Account for each of the results, shown in Fig. P9.87, with a mechanism. In part (a), note
Question:
Account for each of the results, shown in Fig. P9.87, with a mechanism. In part (a), note that the reaction is not observed in the absence of NaOH. In part (b), note that organolithium reagents are strong bases and that the hydrogens on a carbon adjacent to a benzene ring are relatively acidic.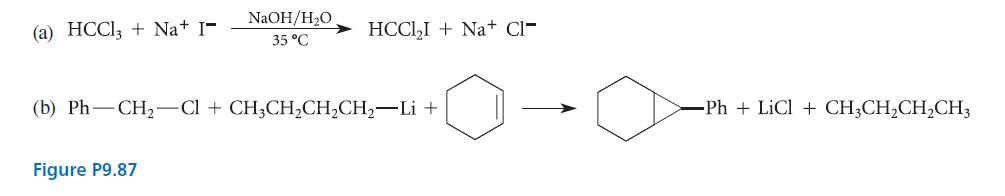
Transcribed Image Text:
(a) HCCl3 + Na+ I- NaOH/H₂O 35 °C Figure P9.87 HCCl₂I+Na+ Cl- (b) Ph-CH₂-Cl + CH3CH₂CH₂CH₂-Li + -Ph+ LiCl + CH3CH₂CH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a Sodium hydroxide acts as a base to form trichloromethyl anion which then for...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Account for each of the following facts with an explanation. 1, 3-Cyclopentadiene is a considerably stronger carbon acid than 1, 4-pentadiene even though the acidic hydrogens in both cases are doubly...
-
In Kekulé's time, cyclohexane was unknown, and there was no proof that benzene must be a six-membered ring. Determination of the structure relied largely on the known numbers of...
-
Recall from Section 9-7 how acetylide ions are alkylated by displacing unhindered alkyl halides. Like acetylide ions, Grignard and organolithium reagents are strong bases and strong nucleophiles....
-
Determine the largest force P that can be exerted at the jaws of the punch without exceeding a stress of 16 ksi on section m-n of the frame.
-
What are the required financial statements for a pension trust fund? What are the required supplementary information schedules?
-
ABC is a partnership owned by Ales, Baker, and Chaplin, who share profits and losses in the ratio of 2:1:1, respectively. The account balances of the partnership at June 30, 2016, follow:...
-
4. Fin and Rho have capital balances of $100,000 and $80,000, respectively, and they share profits equally. The partners agree to accept Che for a 25 percent interest in capital and profits for her...
-
You Can Paint Too prepares and packages paint products. You Can Paint Too has two departments: Blending and Packaging. Direct materials are added at the beginning of the blending process (dyes) and...
-
Jason y Marie estn casados y presentarn una declaracin conjunta. Dirigen un negocio juntos y eligen ser tratados como una empresa conjunta calificada. Su negocio tiene una ganancia neta de $850 por...
-
Provide a curved-arrow mechanism and a rationale for the rearrangement shown in Eq. 10.17. (Use HA as a general abbreviation for the catalyzing acid.) OH 1 CH-CH3 1-cyclobutylethanol acid CH3 + HO...
-
In 1975, a report was published in which the reaction given in Fig. P9.85 was observed. TheOBs (brosylate) group is a leaving group conceptually like halide. (Think of this group as you wouldBr.)...
-
Use the following information about the lions mane jellyfish to answer the questions. The largest species of jellyfish is the lions mane jellyfish. The largest lions mane jellyfish specimen ever...
-
The answer above is NOT correct. The value of (2x + 1)(x + x)dx is
-
Review the resource on organizational theory. Explore the various theories and select one to use for this Discussion. Consider the strengths and limitations of the chosen theory. Compose an analysis...
-
How do the locations of Australian department store Myer affect the ability of the other factors of the operating model canvas (suppliers, organization, processes, and information/management systems)...
-
Critical Reading Review: The Exclusion of Latinos from American Media and History Books Read the article. After reading the article, answer the following questions: 1. What purpose do you think the...
-
1. How does the proposed market segment of residential contracts differ from Smith Electric's current market segment? 2.What does a SWOT analysis tell us about Smith Electric's ability to enter a...
-
Is not sufficient to guarantee the convergence of the alternating series ((-1)n+1an. Alternate the terms of (1/n and ((-1 / n2)? lim an- 0
-
Rosalie owns 50% of the outstanding stock of Salmon Corporation. In a qualifying stock redemption, Salmon distributes $80,000 to Rosalie in exchange for one-half of her shares, which have a basis of...
-
Treatment of a cyclic ketone with diazomethane is a method for accomplishing a ring-expansion reaction. For example, treatment of Cyclohexanone with diazomethane yields Cycloheptanone. Propose...
-
Ketones react slowly with benzeneselenenyl chloride in the presence of HCl to yield ?-phenylselcno ketones. Propose a mechanism for this acid-catalyzed a-substitution reaction. CeHgSeCi Se-C6H5
-
Pentobarbital, marketed under the name Nembutal, is a barbiturate used in treating insomnia. It is synthesized in three steps from diethyl malonate. Show how you would synthesize the dialkylated...
-
Dr. Claudia Gomez, a plastic surgeon, had just returned from a conference in which she learned of a new surgical procedure for removing wrinkles around eyes, reducing the time to perform the normal...
-
QUESTION 2 ( 2 0 Marks ) 2 . 1 REQUIRED Study the information provided below and prepare the Income Statement for the year ended 3 1 December 2 0 2 3 using the marginal costing method. INFORMATION...
-
DROP DOWN OPTIONS: FIRST SECOND THIRD FOURTH 5. Cost of new common stock A firm needs to take flotation costs into account when it is raising capital fromY True or False: The following statement...

Study smarter with the SolutionInn App


