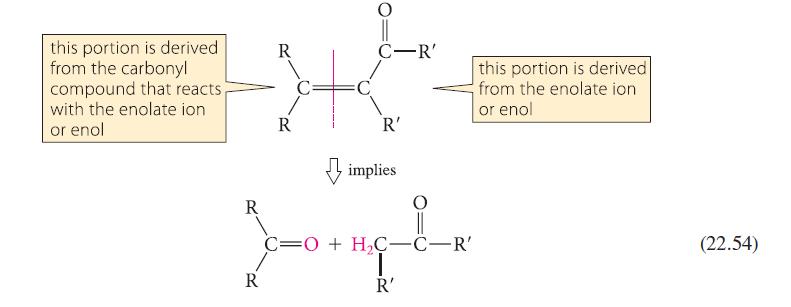
Analyze the aldol condensation in Eq. 22.53 using the method given in Eq. 22.54. Show that four
Question:
Analyze the aldol condensation in Eq. 22.53 using the method given in Eq. 22.54. Show that four possible aldol condensation products might in principle result from the starting material. Explain why the observed product is the most reasonable one.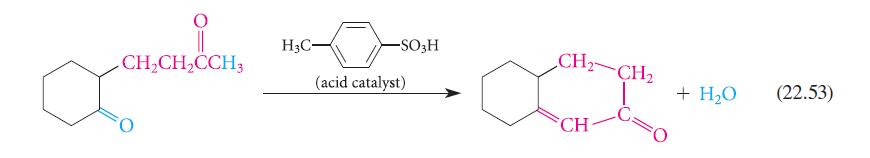
Transcribed Image Text:
CH₂CH₂CCH3 O H3C- -SO3H (acid catalyst) CH₂ CH₂ Ca CH + H₂O (22.53)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
First break the double bond as shown in Eq 2254 to reveal the possible starting material The ...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Q1. How worried are clients and stakeholders in day-to- day product improvement? 2. the industrial corporation Case for Agility "The struggle is not always to the most powerful, nor the race to the...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
A company that makes cola drinks states that the mean caffeine content per 12-ounce bottle of cola is 50 milligrams. You want to test this claim. During your tests, you find that a random sample of...
-
Mountain Cycles uses the FIFO inventory method. Mountain started August with 12 bicycles that cost $42 each. On August 16, Mountain bought 40 bicycles at $68 each. On August 31, Mountain sold 36...
-
What is the employment-at-will doctrine?
-
Explain tariffs, nontariff trade barriers, investment barriers, and government subsidies. What are their main characteristics? How do they differ? LO.1
-
Start with the partial model in the file Ch19 P06 Build a Model.xls on the textbook's Web site. As part of its overall plant modernization and cost reduction program, Western Fabrics' management has...
-
Your company buys a piece of equipment for $ 1 0 , 0 0 0 which has a 1 0 year service life, $ 1 0 0 0 per year maintenance cost starting 1 year after purchase and through the service life of the...
-
The following reaction is known to involve enamine formation between the amine catalyst and the carbonyl marked with an asterisk (*). Draw a curved-arrow mechanism for this reaction, using the...
-
(a) The enzyme KDPG aldolase catalyzes the aldol addition reaction between pyruvate and glyceraldehyde-3-phosphate. The reaction is known to involve the formation of an imine (Schiff base) between a...
-
Brian spent 1/4 of his paycheck to repair his car, and then paid the registration and insurance, which each cost 1/3 of the remainder of his paycheck. If Brian had $0 before he was paid, and he now...
-
Safeway, Inc., operated 1,739 stores as of January 3, 2009. The following data were taken from the company's annual report. All dollar amounts are in thousands. Required a. Compute Safeway's...
-
Rich French, the owner of Rich's Fishing Supplies, is surprised at the amount of actual inventory at the end of the year. He thought there should be more inventory on hand based on the amount of...
-
Carol Lapaz owned a small company that sold boating equipment. The equipment was expensive, and a perpetual system was maintained for control purposes. Even so, lost, damaged, and stolen merchandise...
-
The following footnote related to accounting for inventory was taken from the 2008 annual report of Wal-Mart, Inc. Inventories The Company values inventories at the lower of cost or market as...
-
Plot the magnitude and phase of the frequency response of normalized n-th order lowpass Butterworth filters.
-
Prove Proposition 9.17. A particularly important class of systems are the linear gradient flows in which AT is a symmetric, positive definite matrix. According to Theorem 8.23, all the eigenvalues of...
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
Write a mechanism for the following reaction. (PhCO2)2, heat + CO
-
Hydrogen peroxide and ferrous sulfate react to produce hydroxyl radical (HO), as reported in 1894 by English chemist H. J. H. Fenton. When tert-butyl alcohol is treated with HO generated this way, it...
-
The halogen atom of an alkyl halide can be replaced by the hydrogen atom bonded to tin in tributyltin hydride (Bu3SnH). The process, called dehalogenation, is a radical reaction, and it can be...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


