At 368C the NMR resonances for the ring methyl groups of isopropylmesitylene (protons H a and H
Question:
At 36°8C the NMR resonances for the ring methyl groups of “isopropylmesitylene” (protons Ha and Hb in the following structure) are two singlets at δ 2.25 and δ 2.13 with a 2 : 1 intensity ratio, respectively. When the spectrum is taken at 260°8C, however, it shows three singlets of equal intensity for these groups at δ 2.25, δ 2.17, and δ 2.11. Explain these results.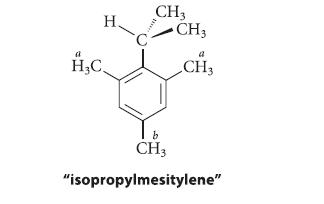
Transcribed Image Text:
H H₂C CH3 CH3 a CH3 CH3 "isopropylmesitylene"
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
At the higher temperature the two ortho methyl groups labeled a i...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Influencer marketing includes only movie, tv and sports celebrities with significant social media audiences. True False 4
-
Histogram. Suppose that the standard input stream is a sequence of double values. Write a program that takes an integer n and two real numbers lo and hi as command-line arguments and uses StdDraw to...
-
At December 31, 2011, Ethan Company Reports the following results for its calendar-year. Cash Sales $1,803,750 Credit Sales 3,534,000 In addition, its unadjusted trail balance includes the following...
-
Astronauts in space cannot weigh themselves by standing on a bathroom scale. Instead, they determine their mass by oscillating on a large spring. Suppose an astronaut attaches one end of a large...
-
Write short notes on: (a) Credit note (b) Debit note (c) Imprest system of petty cash book (d) Contra entry
-
At the beginning of the year, Dillon Company budgeted overhead of $180,000 as well as 15,000 direct labour hours. During the year, Job K456 was completed with the following information: direct...
-
help pls QUESTION 1 During a period when inventory costs are steadily increasing, which of the following is true? Net income will be lower under FIFO than under LIFO. Cost of goods sold will be...
-
A farmer can buy four types of plant food. Each barrel of mix A contains 30 pounds of phosphoric acid, 50 pounds of nitrogen, and 30 pounds of potash; each barrel of mix B contains 30 pounds of...
-
Draw a variation on the rebound hydroxylation mechanism that accounts for the formation of epoxides from some alkenes by CyP450 as shown in Fig. P17.38. C=C +47 +47 Felv Fell S S. Figure P17.38...
-
Parents Ltd acquires 90% of the shares of Sonny Jim Ltd. The following statements of financial position are then drafted. You are to draw up the consolidated statement of financial position. Any gain...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Payroll Payroll Register Register Thomas Avery Towle...
-
Name: Course: Worksheet Lab Experience 5 Logic Circuits (A) Exercise 5.1 Truth table for the example circuit A B Output Value 0 0 1 1 0 1 1 Exercise 5.2 A slight change in the example circuit...
-
Stanley Medical Hospital is a non-profit and a non-chartered hospital planning to acquire several hospitals in the area. The hospital is researching financial options since they want to expand into...
-
Tony and Suzie see the need for a rugged all-terrain vehicle to transport participants and supplies. They decide to purchase a used Suburban on July 1, 2022, for $12,000. They expect to use the...
-
Pacifico Company, a US-based importer of beer and wine, purchased 1,800 cases of Oktoberfest-style beer from a German supplier for 522,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
Under what circumstances might a company have a high p/e ratio even when investors are not optimistic about the company's future prospects?
-
Selected condensed data taken from a recent statement of financial position of Morino Ltd. are as follows. MORINO LTD. Statement of Financial Position (partial) Other current assets...
-
Complete the following reactions. If no reaction is tikety, explain why. (a) (b) CH,SH + NaOH -_, (1 equiv.) 25 C CH OH
-
Outline a synthesis of each ether using either alcohol dehydration or alkene addition, as appropriate. (a) 2-methoxy-2-methylbutane (b) dibutyl ether
-
Give the structure of the that would with mCPBA to give each of the following expoxides. (a) (b) . /A C CH2 H,C C-4 CH,
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)
-
All of the following are roles of a derivative exchange EXCEPT: _____. A) maintaining margin requirements on futures contracts B) reducing the default risk on forward contracts C) performing daily...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...

Study smarter with the SolutionInn App


