Complete the reactions given in Fig. P27.70 assuming the amino acid residue is part of a peptide
Question:
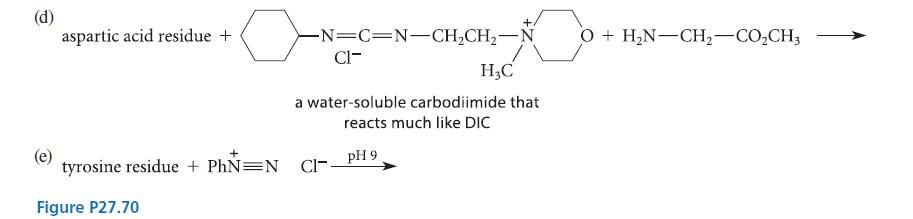
Complete the reactions given in Fig. P27.70 assuming the amino acid residue is part of a peptide in aqueous solution and is at neither the amino nor the carboxy terminus.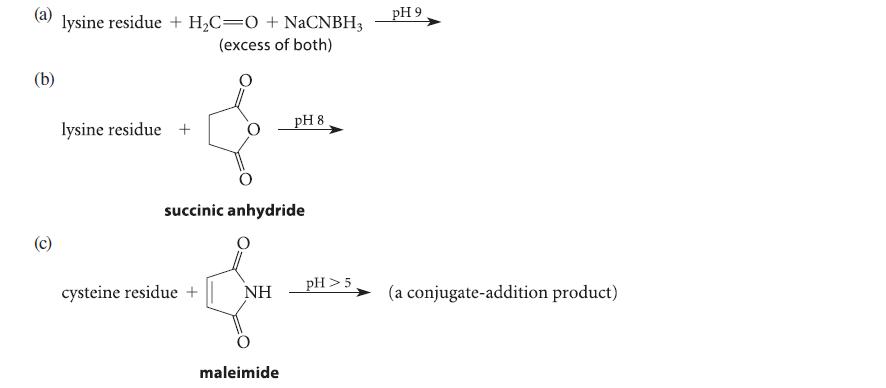
Transcribed Image Text:
(a) lysine residue + H₂C=O + NaCNBH3 (excess of both) (b) (c) lysine residue + succinic anhydride cysteine residue + NH pH 8 maleimide pH > 5 pH 9 (a conjugate-addition product)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a b This is a reductive amination of formaldehyde by the amino group of the lysine residue Because e...View the full answer

Answered By

Niala Orodi
I am a competent and an experienced writer with impeccable research and analytical skills. I am capable of producing quality content promptly. My core specialty includes health and medical sciences, but I can competently handle a vast majority of disciplines.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete the reactions given in Fig. P26.61, assuming the amino acid residue is part of a peptide in aqueous solution and is at neither the amino nor the carboxy terminus. (a) (b) plI 8 lysine...
-
Complete the reactions given in Fig. P19.45 by giving the principal organic product(s). Fig. P19.45 (a) (b) (c) p-toluenesulfonic acid (catalyst CH, t CH,OH - (solvent) ether Hio -caphenone, +...
-
Complete the reactions given in Fig. P22.81 by giving the major organic products. Explain your reasoning. NaOEt excess) EtOH H,o heat CHsI (c) CI NO, H ot, heat C CH LiAIH 2) HaO (e) CH Et CHCHCOEOH...
-
Alert Security Services Co. offers security services to business clients. The trial balance for Alert Security Services Co. has been prepared on the following end-of-period spreadsheet for the year...
-
(Multiple Choice Question) 1. Miga Company and Porter Company both bought a new delivery truck on January 1, 2008. Both companies paid exactly the same cost, $30,000, for their respective vehicles....
-
What is the current trend concerning the use of groups to solve problems and make decisions?
-
A person who is retiring at age 65 and who has $200,000 wants to leave an estate of at least $30,000. How much can the individual draw annually on the $200,000 (starting at the end of the year) if...
-
Carleton Company has two service departments and two production departments. Information on annual manufacturing overhead costs and cost drivers follows: The company allocates service department...
-
The following income statements were drawn from the annual reports of the Atlanta Company and the Boston Company: Atlanta $ 36,100 16.670 19.30 Bonton. $ 88,600 64,520) Net sales Cost of goods sold...
-
Poly-l-lysine (a peptide containing only lysine residues) exists entirely in an a-helical conformation at pH > 11. Below pH 10, however, the peptide becomes a random coil. Poly-l-glutamic acid, on...
-
Complete the reactions given in Fig. P27.75 by giving the structure of the major organic product(s). (a) ethylamine + Ph-N-C=S (b) (d) PhCH=O + KCN + CH3NH, NaOH (1 equiv.) + HNCH-CO; I CH3 CH3...
-
Find the average power absorbed by the 12-y resistor in the network of Problem 14.29 if V (t) = 50 = 25 cos(377t - 45) + 12.5 cos(754t + 45) V.
-
2. (12 points) A researcher hypothesizes that watching Comedy Central reduces anger in prison inmates. A sample of 8 inmates is administered the State Anger/Hostility Scale, their mean score is 5.0...
-
6. A car manufacturer estimates that the cost of production of x cars of a certain model is C(x) = -20x + 0.01 x 2 - 800. How many cars should be produced for a minimum cost? [Please justify your...
-
Latania is a 34-year-old female with a series of volatile interpersonal relationships. She expresses extreme jealousy when her friends spend time with other friends, and she can't maintain romantic...
-
psychological models or psychological theories. Basically these are different ways to think about human behaviorwhat causes behavior and how best to adapt to mental illness or adjustment problems....
-
Product Development: Identify which sports team you are representing and what product you would recommend for the team license. Why did you choose this product? What target audience will purchase it
-
The owner of Warwick Printing, a printing company, is planning direct labor needs for the upcoming year. The owner has provided you with the following information for next year's plans: Each color on...
-
(a) How far away can a human eye distinguish two ear headlights 2.0 m apart? Consider only diffraction effects and assume an eye pupil diameter of 5.0 mm and a wavelength of 550 nm. (b) What is the...
-
Which of the following alkenes can exist as double-bond stereo? Identify the stereo centers in each. (a) CH3CH2CH=====CHCH2CH3 (b) CH,CIH CH-CCH CH
-
Which of the following cannot be, correct formula (r) for an organic compound? Explain (a) C10H20N3 (b)C30H20N2O2 (c) C10H27N3O2 (d) C10H16O;
-
Which compound in each set should have the larger dipole moment? Explain. Propene or 2-methylpropene
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


