Complete the reactions given in Fig. P26.61, assuming the amino acid residue is part of a peptide
Question:
Complete the reactions given in Fig. P26.61, assuming the amino acid residue is part of a peptide in aqueous solution and is at neither the amino nor the carboxy terminus.
(a)
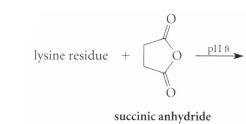
(b)
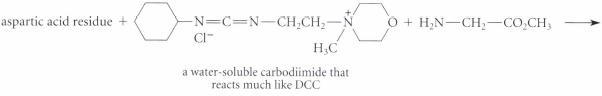
Transcribed Image Text:
plI 8 lysine residue + succinic anhydride
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
a The sidechain amino group of lysine serves as a nucleophile to ...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Using known reactions and mechanisms discussed in the text, complete the reactions given in Fig. P19.46 on p. 942. Fig. P19.46 NaBH4 CH OH
-
Complete the reactions given in Fig. P21.52 by giving the principal organic products. Explain how you arrived at your answers. NaOH CH O (trace) H,C CCHO CH +CH OH (solvent) Ph NH2 1 (CgH,NO3)...
-
Complete the reactions given in Fig. P22.81 by giving the major organic products. Explain your reasoning. NaOEt excess) EtOH H,o heat CHsI (c) CI NO, H ot, heat C CH LiAIH 2) HaO (e) CH Et CHCHCOEOH...
-
As programs become more complex, it becomes increasingly important to plan or "design" your code before writing it. Designing your code will help you organize its logic. It will also help you keep...
-
Predator Pucks, Inc. has current assets of $8,000, net fixed assets of $45,000, current liabilities of $6,800, and long-term debt of $13,800. What is the value of the shareholders' equity account for...
-
Westphal Company's variable selling and administra- tive expenses are 15% of net sales. Fixed expenses are \($60,000\) per quarter. The sales budget shows expected sales of \($200,000\) and...
-
Temptation in consumer choice. Are you willing to pay more for a tempting vice option (e.g., eating a hamburger, surfing the Internet) than a virtuous option (e.g., eating broccoli, reading the...
-
Dunn and Welch both appeared to operate Ruidoso Downs Feed Concession. Dunn sought to obtain credit for Ruidoso Downs from Anderson Hay and Grain Company. Relying on Dunn's financial position,...
-
Suppose you want to purchase a home for $475,000 with a 30-year mortgage at 5.84% interest. Suppose also that you can put down 30%. What are the monthly payments? (Round your answer to the nearest...
-
Brightcove, Inc. acquires all of the stock of Ciber, Inc. for $112.5 million in cash and accounts for the acquisition as a stock acquisition. Balance sheet information at the date of acquisition is...
-
Draw the structure of the major neutral form of each of the following peptides, G-D-G-L-F
-
Show how the acetamidomalonate method can be used to prepare the following unusual amino acids from the indicated starting material and any other reagents. (a) (b) (c) (CH) CDCH CH CO from...
-
Find the length of the curve. y 2 = 4(x + 4) 3 , 0 x 2, y > 0
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
Combustion of a 0.2000-g sample of a compound made up of only carbon, hydrogen, and oxygen yields 0.200 g H 2 O and 0.4880 g CO 2 . Calculate the mass and mass percentage of each element present in...
-
The Pletcher Transportation Company uses a responsibility reporting system to measure the performance of its three investment centers: Planes, Taxis, and Limos. Segment performance is measured using...
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C9H12O (b)C8H10O2 Part (a) TMS 10 O ppm Chemical shift (8) Part (b) TMS O ppm 10 8. Chemical shift (8) Intensity Intensity...
-
Compound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and assign each peak in the NMR spectrum. Note that the absorption at 5.5 ?...
-
Propose a structure for a compound C15H24O that has the following 1H NMR spectrum. The peak marked by an asterisk disappears when D2O is added to thesample. TMS 10 8. 0 ppm Chemical shift (8)...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...

Study smarter with the SolutionInn App


