Propose structures for alcohols that have the following 1H NMR spectra: (a) C9H12O (b)C8H10O2 Part (a) TMS
Question:
Propose structures for alcohols that have the following 1H NMR spectra:
(a) C9H12O
(b)C8H10O2
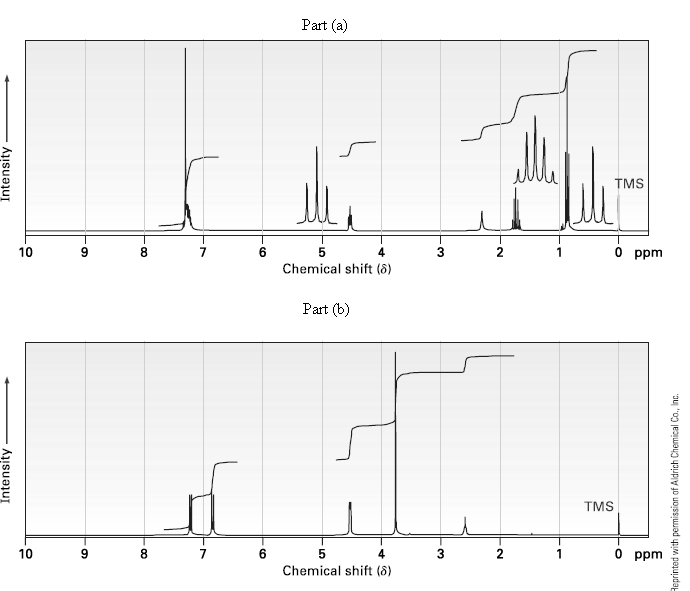
Transcribed Image Text:
Part (a) TMS 10 O ppm Chemical shift (8) Part (b) TMS O ppm 10 8. Chemical shift (8) Intensity Intensity 3. 3. Reprinted with permission of Aldrich Chemical Co., Inc.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a e OH C CHCHCH3 d ba 1Phen...View the full answer

Answered By

BRIAN MUSINGA
I possess a Bachelors of Commerce degree(Marketing option) and am currently undertaking an MBA in marketing. I believe that I possess the required knowledge and skills to tutor in the subject named. I have also written numerous research academic papers much to the satisfaction of clients and my professors.
5.00+
2+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following 1H NMR spectra are for four compounds with molecular formula C6H12O2, Identify the compounds. a. b. c. QUESTION CONTINUE TO NEXT PAGE d. 10 (ppm) frequency 10 (ppm) 10 (ppm)
-
Propose structures for compounds that show the following 1H NMR spectra. (a) C9H13N (b)C15H17N TMS 10 6 5 3 2 O ppm Chemical shift (8) TMS 3 2 O ppm 10 6 1 Chemical shift (8) Intensity Intensity-
-
Assign structures to compounds with the following 1H NMR spectra:? (a) C 4 H 7 ClO? ??IR: 1810 cm ?1 ? (b) C 5 H 7 NO 2 ? ?IR: 2250, 1735 cm ?1 ? (c) C 5 H 10 O 2 ? ? ?IR: 1735 cm ?1 ? TMS O ppm 10...
-
In the Tokyo subway system, routes are labeled by letters and stops by numbers, such as G-8 or A-3. Stations allowing transfers are sets of stops. Find a Tokyo subway map on the web, develop a simple...
-
How can a mission statement be an enduring statement of values and simultaneously provide a basis of competitive advantage?
-
A company showed the following information in its payroll register for the week ended March 16, 2015: 1. Prepare a general journal entry to record the payroll register information. 2. Prepare a...
-
P7-3 Subsidiary purchases parent bonds The separate trial balance for Thanos SA and Merry SA, its 90 percent-owned subsidiary, for the year ended 2014 is as follows: Debits Thanos SA Merry SA Cash...
-
The inventory and sales data for this year for G. Rabbit Company are as follows: Required Using the above data from G. Rabbit Company, compute the following: a. The accounts receivable turnover in...
-
A company has the following sales for the first six months of the year January 1 , 0 1 0 units February 1 , 0 8 7 units March 1 , 1 2 4 units April 1 , 2 1 3 units May 1 , 1 8 5 units June 1 , 1 9 3...
-
Sherron, who is single, purchased a house to use as rental property on April 1, 2008, for $300,000. He moved into the house on June 1, 2018, and used it as a personal residence until August 1. 2019....
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C5H12O (b) C8H10O Part (a) TMS O ppm 10 3 2 Chemical shift (8) Part (b) TMS O ppm 10 8. 6. 4 3 2 Chemical shift (8) Inten
-
Compound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and assign each peak in the NMR spectrum. Note that the absorption at 5.5 ?...
-
Time period. Youre a boating enthusiast. You have recently found the vessel of your dreams, a 20-foot, 500-horsepower piece of nautical delight. However, it costs $55,000 and as of now you only have...
-
20 of 30 Operational, organizational, historical, and custom are all types of need to check on a daily basis as a system administrator. that you'll reporting logging monitoring
-
Consider each of the 3 definitions of "Health" below: 1) "a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity". (WHO) 2) "the ability to...
-
What is the expected FY24 accounts receivable amount and the net plant & equipment amount? Sales Cost of Goods Sold Other Expenses Depreciation Taxable Income Taxes (21%) Net Income FY23 Income...
-
Using research literature in project management, explain the differences between traditional project management from a tactical and strategic perspective. What are the short-term differences? What...
-
The expected value for a question was: E(X) = ( 1 / t h e t a ) ( ( e 5 6 ) / ( and 5 1 ) ) Using the E(X) above, comapre this expected value with the expected value of the Expo(theta) distribution,...
-
Explain how you would develop programs, budgets, and procedures to implement strategic change AppendixLO1
-
What is a make-or-buy decision?
-
Water molecules will form small, stable clusters. Draw one possible water cluster by using six water molecules and maximizing the number of hydrogen bonds for each water molecule.
-
Referring to the retrosynthetic analysis for 2-methylhexane in this section, write reactions for those synthesis routes that are feasible
-
(a) Devise retrosynthetic schemes for all conceivable alkynide anion alkylation syntheses insect pheromones undecane and 2-methylheptadecane. (b) Write reactions for two feasible syntheses of each...
-
Each of the following names is incorrect. Give the correct name and explain your reasoning. (a) trans-3-Pentene (b) 1, 1-Dimethylethene (c) 2-Methylcyclohexene (d) 4-Methylcyclobutene (e)...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


