Consider the bromine addition shown in Figure P15.69. Product A is the predominant product formed at low
Question:
Consider the bromine addition shown in Figure P15.69. Product A is the predominant product formed at low temperature. If the products are allowed to stand under the reaction conditions or are brought to equilib-rium at higher temperature, product B is the only product formed.
(a) Which is the kinetic product and which is the thermodynamic product?
(b) Give a structural reason that the thermodynamic product is more stable than the kinetic product.
(c) Propose a mechanism that explains why the kinetic product is formed more rapidly even though it is less stable. (Hint: The rate-limiting step of bromine addition is formation of the bromonium ion.)
(d) Propose a mechanism for the equilibration of the two compounds that does not involve the alkene starting material.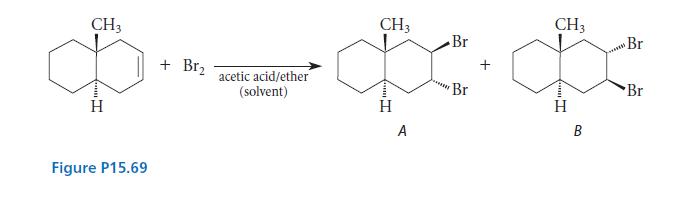
Step by Step Answer:






