(a) Which carbocation is more stable: the carbocation formed by protonation of isoprene at carbon-1 or the...
Question:
(a) Which carbocation is more stable: the carbocation formed by protonation of isoprene at carbon-1 or the carbocation formed by protonation of isoprene at carbon-4? Explain.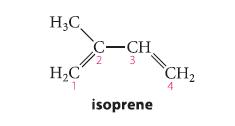
(b) Predict the products expected from the addition of one equivalent of HBr to isoprene; explain your reasoning.
(c) Predict the products expected from the addition of one equivalent of HBr to trans-1,3,5-hexatriene; explain your reasoning.
(d) In parts (b) and (c), which are likely to be the kinetically controlled products and which are likely to be the thermodynamically controlled ones? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





