Determine whether the following ,-unsaturated ketone can be prepared by an aldol reaction. H3C c= C-CHCH3 H
Question:
Determine whether the following α,β-unsaturated ketone can be prepared by an aldol reaction.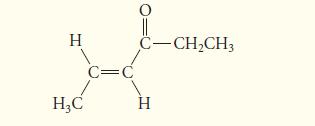
Transcribed Image Text:
н H3C c= C-CH₂CH3 H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
Following the procedure in Eq 2254 analyze the desired product as follows The desired product requir...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4278+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A ketone can be prepared from the reaction of a nitrile with a Grignard reagent. Describe the intermediate that is formed in this reaction, and explain how it can be converted to a ketone.
-
Each of the following substances can be prepared by a nucleophilic addition reaction between an aldehyde or ketone and a nucleophile. Identify the reactants from which each was prepared. It the...
-
(a) The insecticide DDT can be prepared by the reaction shown in Fig. P19.60a. Remembering that a protonated aldehyde or ketone is a type of carbocation, and that carbocations are electrophiles, draw...
-
The SEC Form 10-K of Google is reproduced online at www.wiley.com/college/pratt. REQUIRED: Review the Google 2012 SEC Form 10-K, and answer the following questions: a. Compute Googles long-term debt...
-
The adjusted trial balance of Daddys Music Company at April 30, 2012, follows: Requirements 1. Journalize Daddys closing entries. 2. Prepare Daddys single-step income statement for the year. 3....
-
The elimination of solicitor-client privilege would significantly undermine the integrity of the Canadian legal system. True or false? Explain your answer.
-
Discuss the key points in the debate over whether a business has an optimal capital structure.
-
What would happen to the sampling distribution of the mean if we increased sample size from 5 to 25?
-
Many firms acquire other privately or publicly traded companies or divisions of companies to increase value to the residual owners (the stockholders). Explain how you go about to estimate the Fair...
-
Give the structures of (a) Diethyl malonate (pK a = 12.9) (b) Ethyl acetoacetate (ethyl 3-oxobutanoate, pK a = 10.7), identify the acidic hydrogen in each, and explain why these compounds are much...
-
Give the structures of the aldol addition products expected from the base-catalyzed reaction of acetaldehyde and propionaldehyde.
-
Evaluate the integral. 1/4 sect dt
-
Prove that Eq. (19.34) gives the simplest multi-gluon and gluon-quark states that contain an \(\mathrm{SU}(3)\) color singlet in the decomposition. Data from Eq. 19.34 (GG)1: (88)1 (Gqq) : [8 (383)8]...
-
In question 70, what is the probability that of the 100 cars test-driven, more than 35 cars get more than 45 miles per gallon? How many of the 100 cars tested would you expect to get more than 45...
-
Construct the braid group products (a) (b) using the algorithm of Fig. 29.16 . Data from Fig. 29.16
-
Worksheet The adjusted trial balance columns of a worksheet for Bond Corporation are shown below. The worksheet is prepared for the year ended December 31. Complete the worksheet by (a) entering the...
-
The Healthy Catering Service had the following transactions in July, its first month of operations: 1 Kelly Foster contributed \(\$ 18,000\) of personal funds to the business in exchange for common...
-
Convert the second order coupled system of ordinary differential equations into a first order system involving four variables. = a+bu+cu+dv,
-
Tiger, Inc. signed a lease for equipment on July 1, 2007.The lease is for 10 years (the useful life of the asset).The first of 10 equal annual payments of $500,000 was made on July 1, 2007.The...
-
Write a detailed mechanism for the following reaction. Br OH HBr
-
(a) What factor explains the observation that tertiary alcohols react with HX faster than secondary alcohols? (b) What factor explains the observation that methanol reacts with HX faster than a...
-
Assign oxidation states to each carbon of ethanol, acetaldehyde, and acetic acid. [Ol Ethanol Acetaldehyde Acetic acid
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


