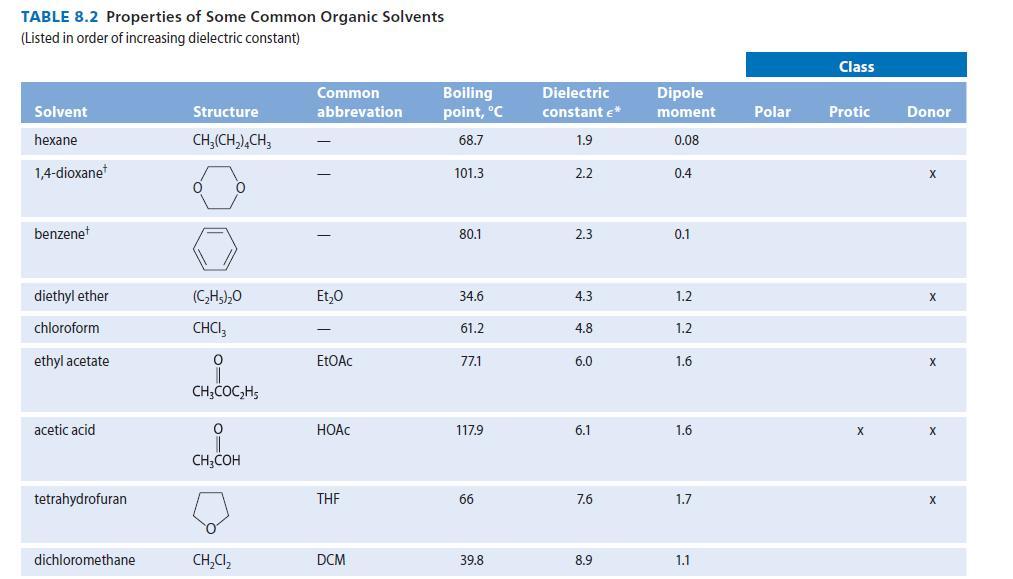
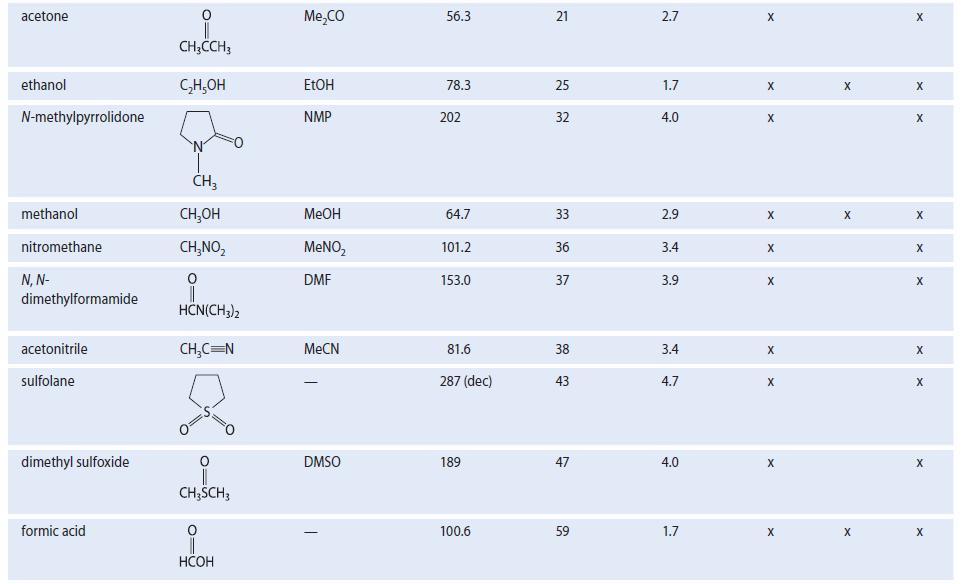
Dimethyl sulfoxide (DMSO, Table 8.2) has a very large dipole moment (4.0 D). Using structures, show the
Question:
Dimethyl sulfoxide (DMSO, Table 8.2) has a very large dipole moment (4.0 D). Using structures, show the stabilizing interactions to be expected between DMSO solvent molecules and
(a) A dissolved sodium ion;
(b) Dissolved water;
(c) A dissolved chloride ion.

Transcribed Image Text:
TABLE 8.2 Properties of Some Common Organic Solvents (Listed in order of increasing dielectric constant) Solvent hexane 1,4-dioxane benzenet diethyl ether chloroform ethyl acetate acetic acid tetrahydrofuran dichloromethane Structure CH₂(CH₂), CH3 0 (C₂H5)₂0 CHCI 0 CH3COC₂H5 0 CH3COH CH₂Cl₂ Common abbrevation Et₂0 EtOAc HOAC THE DCM Boiling point, °C 68.7 101.3 80.1 34.6 61.2 77.1 117.9 66 39.8 Dielectric constant €* 1.9 2.2 2.3 4.3 4.8 6.0 6.1 7.6 8.9 Dipole moment 0.08 0.4 0.1 1.2 1.2 1.6 1.6 1.7 1.1 Polar Class Protic X Donor X X X X X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
1 a Below are represented donor interactions DMSO molecules function as Lewi...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Acetone (Table 8.2) has a significant dipole moment (2.7 D). Using structures, show the stabilizing interactions to be expected between acetone solvent molecules and (a) A dissolved potassium ion;...
-
A vessel filled with gas is divided into two equal parts I and 2 by a thin heat-insulating partition with two holes. One hole has a small diameter, and the other has a very large diameter (in...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
(1) How Does Strategy Respond to Environmental Factors Imprima Corporation is based in the United States and is examining the prospects for expanding into international markets with its main product,...
-
Is it really fair to assume that all business people are aware of these rules about exporting controlled items? What if an honest businessperson actually does not know about the laws?
-
A steel beam ABC is simply supported at A and B and has an overhang BC of length L = 150 mm (see figure). The beam supports a uniform load of intensity q = 4.0 kN/m over its entire span AB and 1.5q...
-
Sustainability is becoming an important component in both Inditex and its competitors marketing strategy, and it has in turn changed the way that productionand therefore the companies global supply...
-
The inventory of Royal Decking consisted of five products. Information about the December 31, 2011, inventory is as follows: Disposal costs consist only of a sales commission equal to 10% of selling...
-
question Findings: -Affecting operations -Affecting sales revenue -Affecting profit margin Case study: ABC Limited is an established manufacturer of a line of electric fans targeting both the...
-
Into a separatory funnel is poured 200 mL of dichloromethane (density = 1.33 g mL 1 ) and 55 mL of water. This mixture forms two layers. One milliliter of 2-octanol is added, and the mixture is...
-
In each case, which distribution has the higher entropy? Explain. (a) Four coins in which two are heads and two are tails, or four coins in which one is heads and three are tails. (b) Six coins in...
-
A survey of the morning beverage market shows that the primary breakfast beverage of 17% of Americans is milk. A Canadian dairy company believes the figure is higher in Canada. The company contacts a...
-
Name and define the more common constraints in any given project.
-
Graph the function f(x)=-x+4x-20 State where f(x) is increasing and decreasing. State any absolute extrema (if they exist). Determine the Domain and Range.
-
A residential wiring circuit is shown in the figure. In thismodel, the resistor R 3 is used to model a 250 V appliance(such as an electric range), and the resistors R 1 and R 2 are used to model 125...
-
1. The speed limit on some interstate highways is roughly 100 km/h. (a) What is this in meters per second? (b) How many miles per hour is this? 2. A car is traveling at a speed of 33 m/s. (a) What is...
-
Questions 33 and 34 are based on the following information: Bilog Company's budgeted fixed overhead costs are P50,000 and mthe variable factory overhead rate is P4 per direct labor hour. The standard...
-
People who inherit familial periodic paralysis often develop very low blood potassium concentrations. How would you explain that the paralysis may disappear quickly when potassium ions are...
-
Arlington Merchants reported the following on its income statement for the fiscal years ending December 31, 2016 and 2015. 2016 2015 Sales $4,857,500 $4,752,900 Cost of goods sold 3,258,950 3,207,000...
-
Predict the multiplicities of the absorptions for the hydrogen's of these groups, assume that hydrogen's labeled a are different from those labeled x but that all of those labeled a are identical and...
-
Predict the 1H.NMR spectra of these compounds include the approximate chemical shift, multiplicity, and integral for each type ofhydrogen. CI b) CH;CHCH; ) C,CH,H c) CH,CH,OCH,CH3 CH2CH2NO2 f)...
-
How many different absorption bands would appear in the 13C-NMR spectra of thesecompounds? b) CH;CH,CH,CH,CH3 c) CH CCH,CH;CH,CH, d)
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


