Draw the structure of the major species present in solution when 0.01 mole of the following acid
Question:
Draw the structure of the major species present in solution when 0.01 mole of the following acid in aqueous solution is treated with 0.01 mole of NaOH. Explain.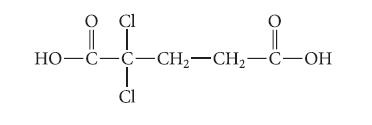
Transcribed Image Text:
O Cl O i OH HO-C-C-CH₂-CH₂-C-OH T CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Only enough NaOH is present to ionize one of the carboxy ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the structure of an optically active triglyceride containing one equivalent of stearic acid and two equivalents of oleic acid. Draw the products expected when this triglyceride reacts with the...
-
When d-glucose is treated with an aqueous bromine solution (buffered to a pH of 6), an aldonic acid is formed called d-gluconic acid. Treatment of d-gluconic acid with an acid catalyst produces a...
-
When the (R, R) isomer of the amine shown is treated with an excess of methyl iodide, then silver oxide, then heated, the major product is the Hofmann product. (a) Draw the structure of the major...
-
Jack Hammer invests in a stock that will pay dividends of $2.00 at the end of the first year; $2.20 at the end of the second year; and $2.40 at the end of the third year. Also, he believes that at...
-
Suppose KC Toys buys $185,800 worth of MegoBlock toys on credit terms of 2/10, n/30. Some of the goods are damaged in shipment, so KC Toys returns $18,530 of the merchandise to MegoBlock. Requirement...
-
What is the days' sales in accounts receivable ratio?
-
Can you think of at least one practical problem of adopting an SVA approach?
-
Jordan Auto has developed the following production plan for its new auto part. Each unit contains three pounds of raw material. The desired raw materials ending inventory is 120 percent of the next...
-
Question #6 (Short Answer) The following represents an issue/procedure an auditor found during an engagement. Please identify (1) what audit objective the auditor is attempting to address (2) what...
-
Draw the structures and give the names of all the dicarboxylic acids with the formula C 6 H 10 O 4 . Indicate which are chiral, which would readily form cyclic anhydrides on heating, and which would...
-
Give the product expected when butyric acid (or other compound indicated) reacts with each of the following reagents. (a) Ethanol (solvent), H 2 SO 4 catalyst (b) Aqueous NaOH solution (c) LiAlH 4...
-
Apple and Samsung have two pricing strategies: Set a high (monopoly) price or set a low (competitive) price. Suppose that if they both set a competitive price, economic profit for both is zero. If...
-
Lennys Limousine Service (LLS) is considering the purchase of two Hummer limousines. Various information about the proposed investment follows: Required: Help LLS evaluate this project by calculating...
-
Lancer Corp. has the following information available about a potential capital investment Required: 1. Calculate the projects net present value. 2. Without making any calculations, determine whether...
-
Woodchuck Corp. is considering the possibility of outsourcing the production of upholstered chair pads included with some of its wooden chairs. The company has received a bid from Padalong Co. to...
-
Woodchuck Corp. is considering eliminating a product from its line of outdoor tables. Two products, the Oak-A and Fiesta tables, have impressive sales. However, sales for the Studio model have been...
-
Suppose that Flyaway Company also produces the Windy model fan, which currently has a net loss of \($40,000\) as follows: Eliminating the Windy product line would eliminate \($20,000\) of direct...
-
Suppose you know all eigenvalues of a matrix as well as their algebraic and geometric multiplicities. Can you determine the matrix's Jordan canonical form?
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
Furan possesses less aromatic character than benzene as measured by their resonance energies (96 kJ mol-1 for furan; 151 kJ mol-1 for benzene). What reaction have we studied earlier that shows that...
-
If benzene were 1,3,5-cyclohexatriene, the carbon-carbon bonds would be alternately long and short as indicated in the following structures. However, to consider the structures here as resonance...
-
For each of the pairs below, predict specific aspects in their 1H NMR spectra that would allow you to distinguish one compound from the other. (a) (b) (c) Br Br
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


