Each of four bottles, A, B, C, and D, is labeled only C 6 H 12
Question:
Each of four bottles, A, B, C, and D, is labeled only “C6H12” and contains a colorless liquid. You have been called in as an expert to identify these compounds from their spectra:
Compound A:
NMR: one line only at δ 1.66 (s); IR: no absorption in the range 1620–1700 cm–1; reacts with Br2 in CCl4
Compound B:
IR: 3080, 1646, 888 cm–1; NMR spectrum in Fig. P13.45a
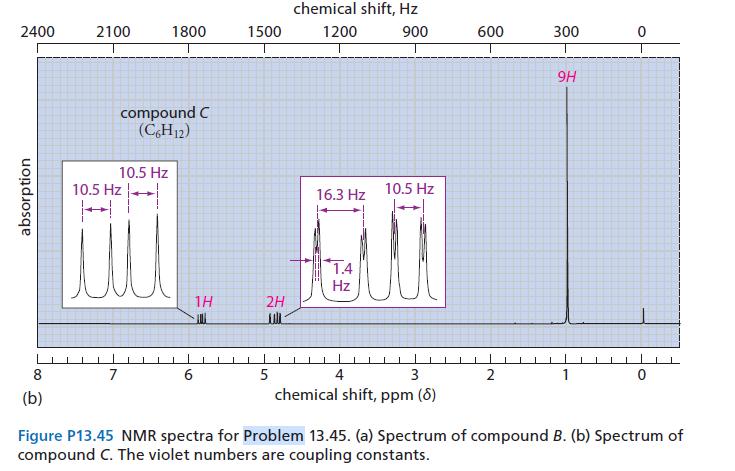
Compound C:
IR: 3090, 1642, 911, 999 cm–1; NMR spectrum in Fig. P13.45b
Compound D:
NMR: one line only at δ 1.40 (s); does not react with Br2 in CCl4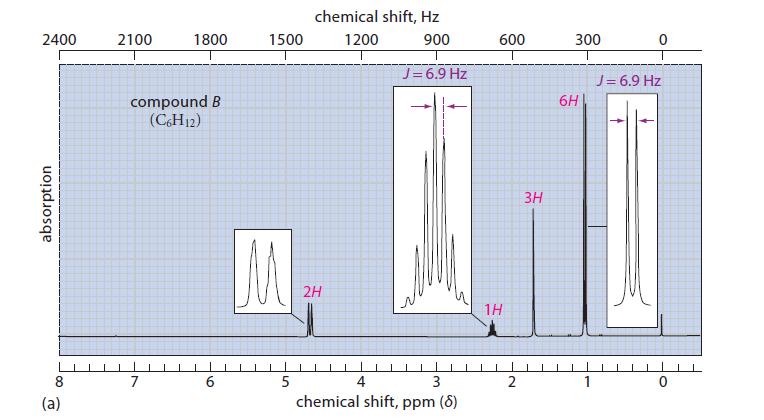
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





