Question: Amines are converted into alkenes by a two-step process called the Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the
Amines are converted into alkenes by a two-step process called the Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it undergoes readyelimination.
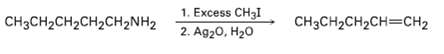
1. Excess CH3I CH3CH2CH2CH=CH2 CH3CH2CH2CH2CH2NH2 2. Ag20, H20
Step by Step Solution
3.51 Rating (181 Votes )
There are 3 Steps involved in it
excess CH CHCHCHCHCHNCH33 CHCHCHCHCHNCHTA90 HO CH3CHCHCHCH N... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-E-R (164).docx
120 KBs Word File


