Tell whether the following compounds are ortho-, meta-, orpara-di-substituted: (a) CI. CH3 (b) NO2 (c) SO3H Br
Question:
Tell whether the following compounds are ortho-, meta-, orpara-di-substituted:
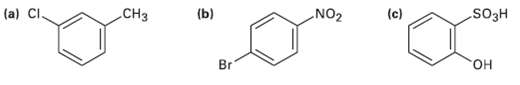
Transcribed Image Text:
(a) CI. CH3 (b) NO2 (c) SO3H Br он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
An ortho disubstituted benzene has two substituents in a 12 ...View the full answer

Answered By

Patrick Busaka
I am a result oriented and motivated person with passion for challenges because they provide me an opportunity to grow professionally.
5.00+
38+ Reviews
58+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Tell whether the following compound is chiral. trans- I,2-dimethylcyclopropane
-
The following compounds are only slightly soluble in water, but one of them is very soluble in a dilute aqueous solution of sodium hydroxide. The other is still only slightly soluble. (a) Explain...
-
The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. (a) Show the structure of the conjugate base of each acid, including any...
-
A ping pong ball is drawn at random from an urn consisting of balls numbered 4 through 9. A player wins $1.5 if the number on the ball is odd and loses $1.5 if the number is even. Let x be the amount...
-
If you were advising a North Dakota company about its selection process, would you advise it to relax its selection criteria during the oil boom? Why or why not?
-
Write the answers to the following question in the space provided. 1. What is the original cost of asset number 120 (Veigel G350 Van) as shown on the fixed assets list? 2. What is the salvage value...
-
Describe some career possibilities in theme parks. LO.1
-
Howser Companys fixed overhead costs are $4 per unit, and its variable overhead costs are $8 per unit. In the first month of operations, 50,000 units are produced, and 48,000 units are sold. Write a...
-
which of the following subsequent expenditures would be capitalized
-
Dan and Susan are faced with an important decision. After having discussed different financial scenarios, the two computer engineers felt it was time to finalize their cash flow projections and move...
-
Amines are converted into alkenes by a two-step process called the Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2...
-
Give IUPAC names for the followingcompounds: CH NH2 (a) Cl Br (b) (c) CH2CH2CHCH3 Br (f) CH (e) (d) CI. CH H CH-CH O2N" NO2 "C
-
Melford Hospital operates a general hospital, but rents space and beds to separately owned entities rendering specialized services such as pediatrics and psychiatry. Melford charges each separate...
-
Explain the principles of database normalization and denormalization, delineating their respective roles in optimizing data storage efficiency, query performance, and data integrity in relational...
-
Asymptotic Computational Complexity O(): Calculate the time complexity of each function below and explain your reasoning. Write your answers on paper and submit a scanned copy. (5 pts each) def...
-
Happy Valley Software has developed a new meteorology software package that will likely revolutionize the weather forecasting industry. They are looking to market the software to the following three...
-
Please read the essay Nasty Women Have Much Work To Do from Alexandra Petri on pages 45-47. In your discussion post, please share your thoughts on what specific strategies she uses to create tone and...
-
We live in an increasingly hyper-competitive global marketplace, where firms are fighting to stay lean and flexible in an effort to satisfy increasingly diverse and specialized consumer demand. In...
-
outline common methods of collecting overdue debts;
-
Refer to the Conservation Ecology (Dec. 2003) study of the causes of forest fragmentation, presented in Exercise 2.166 (p. 97). Recall that the researchers used advanced high-resolution satellite...
-
Explain how you might deduce the equilibrium constant for a reaction in which you know the initial concentrations of the reactants and products and the equilibrium concentration of only one reactant...
-
Propose a mechanism for the base-catalyzed epimerization of erythrose to a mixture of erythrose and threose.
-
Show how another enediol rearrangement can move the carbonyl group from C2 in fructose to C3.
-
Show how another enediol rearrangement can move the carbonyl group from C2 in fructose to C3. Discuss.
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...

Study smarter with the SolutionInn App


