Question: Give IUPAC names for the followingcompounds: CH NH2 (a) Cl Br (b) (c) CH2CH2CHCH3 Br (f) CH (e) (d) CI. CH H CH-CH O2N NO2
Give IUPAC names for the followingcompounds:
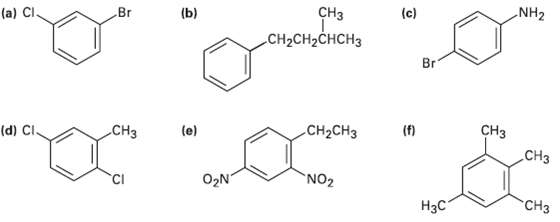
CH NH2 (a) Cl Br (b) (c) CH2CH2CHCH3 Br (f) CH (e) (d) CI. CH H CH-CH O2N" NO2 "C
Step by Step Solution
3.42 Rating (171 Votes )
There are 3 Steps involved in it
Remember to give the lowest possible numbers to substi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-B (77).docx
120 KBs Word File


