Explain how you would distinguish each of the following isomeric compounds from the others using NMR spectroscopy.
Question:
Explain how you would distinguish each of the following isomeric compounds from the others using NMR spectroscopy. Be explicit.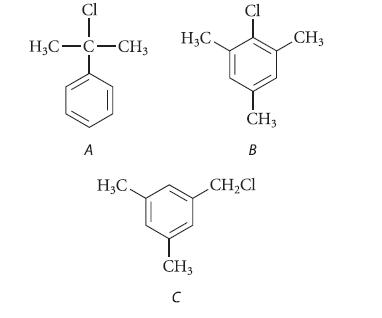
Transcribed Image Text:
Cl T H3C-C-CH3 A H₂C. H₂C. CH3 C Cl CH3 B CH₂Cl CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Only compound A should have a proton NMR spectrum containi...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How could 1H NMR distinguish between the compounds in each of the following pairs? a. CH3CH2CH2OCH3 and CH3CH2OCH2CH3 b. BrCH2CH2CH2Br and BrCH2CH2CH2NO2 c. d. e. f. g. h. i. CH3 CH, CHa CH3CH CHC...
-
How would you distinguish among the compounds within each of the following sets using their NMR spectra? Explain carefully and explicitly what features of the NMR spectrum you would use. (a)...
-
How would you differentiate between the compounds in each of the following pairs? (a) p-ethylbenzoic acid and ethyl benzoate by IR spectroscopy (b) N-methylpropanamide and N-ethyl acetamide by proton...
-
Suppose that the Medicare rate of hospital reimbursement is reduced. Explain why the costs may not be shifted to other patients in the short run.
-
Calculation of tax rafts A multinational computer equipment manufacturer reported the following amounts for t recent years 4in millions of U.S. dollars). The firm applies U.S. GAAP. a. Compute the...
-
A ring of radius R has total charge Q. a. At what distance along the z-axis is the electric field strength a maximum? b. What is the electric field strength at this point?
-
True or False Quiz Assuming that no questions are left unanswered, in how many ways can a six-question true-false quiz be answered? Classical Probabilities In Exercises 2934, a probability experiment...
-
Why can undue emphasis on labor efficiency variances lead to excess work in process inventories?
-
If you work after age 64, your social security benefits will increase by one-fourth of one percent for each month you delay retirement up until what age
-
(a) Arrange the three isomeric dichlorobenzenes in order of increasing dipole moment (smallest first). (b) Assuming that the dipole moment is the principal factor governing their relative boiling...
-
Which of the following compounds cannot contain a benzene ring? How do you know? C10H16 A C8H6Cl B C5H4 C C10H15N D
-
Does the center of pressure change with airspeed and AoA?
-
According to a recent study, 21% of American college students graduate with no student loan debt. Suppose we obtain a random sample of 106 American college students and record whether or not they...
-
Differentiate the following with respect to x: a. y=5x+2x + x + 15 b. y=4x+3x - 4x - 10 c. y = 3Sin(5x) d. y = 3Cos(3x) e. y=10e -25x f. y = log(6x)
-
Question 2. The rate of drug destruction by the kidneys is proportional to the amount of the drug in the body. The constant of proportionality is denoted by K. At time t the quantity of the drug in...
-
5. 6. -1 (4a) U u X2 1 X2 -2 x -1 -2 12 (4b) U -2 2 Y y 16 x2 X2 3 1 (4c) U u - x 2 Y y -8 Y y -20 5 x X2 2 Find the state space models of the three systems shown in Fig. 4a, Fig. 4b, and Fig. 4c,...
-
Given the following data for Mehring Company, compute total manufacturing costs, prepare a cost of goods manufactured statement, and compute cost of goods sold. Direct materials used $230,000...
-
Gosling Company determines its annual income tax expense to be $459,000. Of that amount, $300,000 has already been paid during the year (on a quarterly basis) and charged to the Income Taxes Expense...
-
Gordon and Lisa estimate that they will need $1,875,000 in 40 years for their retirement years. If they can earn 8 percent annually on their funds, how much do they need to save annually?
-
Using the Huckel 4n + 2 rule, determine whether each of the following compounds is likely to be aromatic. Explain how you arrived at the -electron count in each case. (a) (b)
-
The following compound is not aromatic even though it has 4n + 2 electrons in a continuous cyclic array. Explain why this compound is not aromatic.
-
Rank the isomers within each set in order of increasing heat of formation (lowest first). CH-CH,
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


