Which of the following compounds cannot contain a benzene ring? How do you know? C10H16 A C8H6Cl
Question:
Which of the following compounds cannot contain a benzene ring? How do you know?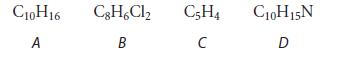
Transcribed Image Text:
C10H16 A C8H6Cl₂ B C5H4 C C10H15N D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
A compound containing a benzene ring must have at least six carbon atoms and four ...View the full answer

Answered By

Ashish Jaiswal
I have completed B.Sc in mathematics and Master in Computer Science.
4.90+
20+ Reviews
39+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds below do you expect to have a longer λ max ?
-
Which of the following compounds would you expect to be the most generally reactive, and why? DO
-
Which of the following compounds are suitable solvents for Grignard reactions? (a) n-hexane (b) CH3- O-CH3 (c) CHCl3 (d) cyclohexane (e) benzene (f) CH3OCH2CH2OCH3
-
Discuss the ways that managed care organizations can infl uence the adoption of new technologies.
-
Classification and interpreting income statements Dyreng Plc. (Dyreng), a Belgian-based construction firms, reported the following information for 2008. Dyreng applies IFRS and reports in thousands...
-
A plastic rod with linear charge density is bent into the quarter circle shown in FIGURE P23.48. We want to find the electric field at the origin. a. Write expressions for the x-and y-components of...
-
Event A: rolling a 2
-
Refer to the information in Exercise 9- 6 to complete the following requirements. a. On February 1 of the next period, the company determined that $ 6,800 in customer accounts is uncollectible;...
-
answers? 1. [10 pts) Epek Hobbs, Inc. is exdering the abilities of as maintenance services division. Opening the division will require a new building staff anders. The proposed plan involves...
-
Explain how you would distinguish each of the following isomeric compounds from the others using NMR spectroscopy. Be explicit. Cl T H3C-C-CH3 A HC. HC. CH3 C Cl CH3 B CHCl CH3
-
Give the products expected (if any) when ethylbenzene reacts under the following conditions. (a) Br in CCl4 (dark) (c) concd. HSO4 O (d) (b) HNO3, HSO4 Et-C-Cl, AlCl3 (1.1 equiv.), then HO (e) CHBr,...
-
In emergencies with major blood loss, the doctor will order the patient placed in the Trendelenburg position, in which the foot of the bed is raised to get maximum blood flow to the brain. If the...
-
Solve for "C" and "E": 1) E cos (15)-C=0 2) -300+ C+E sin (15) = 0
-
Let u=3, b. Compute uv, uv, 2-3 v =
-
Using Complex Numbers show that d cosz=-sinz dz
-
use for loops to solve the following problems 1. Write a complete C++ program that does the following. It asks the user to enter their age (which is assumed to be a positive integer). The program...
-
Profile Vickers hardness test Penetrating body: Square diamond pyramid :Test force F N ... 981 N (HV 5 ... HV 100) 49 :Measured value Diagonals of the square impression d Hardness value: F 0,189 F...
-
Fox Company has debt totaling $2,000,000 and total stockholders' equity of $4,000,000. Wolfe Company has debt totaling $3,000,000 and stockholders' equity of $5,000,000. a. Calculate the debt ratio...
-
How will relating product contribution margin s to the amount of the constrained resource they consume help a company maximize its profits?
-
Assume you have unlabeled samples of the compounds within each of the following sets. Explain how UV-vis spectroscopy could be used to distinguish each compound in the set from the other (s). (a) (b)...
-
How would the color of -carotene (structure on p. 689) be affected by treatment of the compound with a large excess of H2 over a Pt/C catalyst? Explain.
-
(a) Draw line-and-wedge structures for the two enantiomers of the following allene. (b) One enantiomer of this compound has a specific rotation of - 30.7o. What is the specific rotation of the other?...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


