Give the products expected (if any) when ethylbenzene reacts under the following conditions. (a) Br in CCl4
Question:
Give the products expected (if any) when ethylbenzene reacts under the following conditions.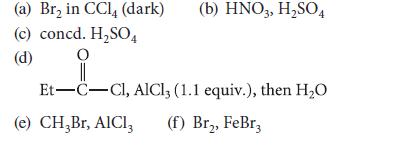
Transcribed Image Text:
(a) Br₂ in CCl4 (dark) (c) concd. H₂SO4 O (d) (b) HNO3, H₂SO4 Et-C-Cl, AlCl3 (1.1 equiv.), then H₂O (e) CH₂Br, AlCl3 (f) Br₂, FeBr3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
In part e ethylbenzene must be present in large excess for the monosubstitution produ...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the products expected when the following compounds are ozonized and reduced. (a) (b) (c) (d)
-
(a) Give the products expected when acetic formic anhydride reacts with (i) aniline and (ii) benzyl alcohol. (b) Propose mechanisms for these reactions.
-
(a) Give the products expected when (+)-glyceraldehyde reacts with HCN. (b) What is the relationship between the products? How might they be separated? (c) Are the products optically active? Explain.
-
In the Akerlof example, the individuals are treated as indifferent to risk. What would you expect to see in these markets if individuals wanted to avoid risk? What if there were some risk lovers?
-
Classification and interpr4eting income statements SeaBreeze Inc., a Taiwan-based semiconductor manufacturer, reported the following information for 2008. SeaBreeze Inc. applies IFRS and reports in...
-
A sphere of radius R and surface charge density is positioned with its center distance 2R from an infinite plane with surface charge density . At what distance from the plane, along a line toward...
-
Event B: rolling a 10
-
Namaka Inc. (Namaka) recently purchased new display cases for its retail stores. The display cases cost $150,000, taxes were $22,000 (of which $19,500 is refundable), delivery cost $5,000, and set-up...
-
Answers to each of the five (5) situations described below addressing the required criteria (i.e. 1 & 2) in each independent case. Mark Calaway has been appointed as a junior auditor of West...
-
Which of the following compounds cannot contain a benzene ring? How do you know? C10H16 A C8H6Cl B C5H4 C C10H15N D
-
Using benzene and any other reagents, outline a synthesis of each of the following compounds. (a) Cyclohexylcyclohexane (b) Tert-butylcyclohexane
-
Working individually or in groups, bring several business publications such as Business Week and the Wall Street Journal to class. Based on their content, compile a list entitled, What HR Managers...
-
Determine dy/dr when 3x+4y = 3.
-
Problem 3. Doping a Semiconductor The following chemical scheme is used to introduce P-atoms as a dopant into a semiconductor - a silicon chip. POCI3 Cl POCI 3 vapor P P SiO2 + P(s) CVD coating Si...
-
The system shown in the following figure is in static equilibrium and the angle is equal to 34 degrees. Given that the mass1 is 8 kg and the coefficient of static friction between mass1 and the...
-
Pre-Writing step for a report for your boss on Richard Hackman's statement that using a team to complete a complex project may not be the best approach. Review your classmates' contributions to the...
-
For the graph of the equation x = y - 9, answer the following questions: the x- intercepts are x = Note: If there is more than one answer enter them separated by commas. the y-intercepts are y= Note:...
-
Pearl Company sells $1,000,000 general obligation bonds for 101. The interest rate on the bonds, paid quarterly, is 5 percent. Calculate (a) The amount that the company will actually receive from the...
-
How can NAFTA be beneficial to suppliers of Walmart?
-
The following natural product readily gives a Diels-Alder adduct with maleic anhydride (structure in Eq. 15.12a, p. 698) under mild conditions. What is the most likely configuration of the two double...
-
Explain why 4-methyl-1, 3-pentadiene is much less reactive as a diene in Diels-Alder reactions than (E)-1, 3-pentadiene, but its reactivity is similar to that of (Z)-1, 3-pentadiene.
-
This problem describes the result that established the intrinsic preference for L, Z-addition in the reaction of hydrogen halides with conjugated dienes. (a) What is the relationship between the...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


