Explain the position of substitution observed in the bromination of thiophene-3-carboxylic acid shown in Eq. 26.18. Draw
Question:
Explain the position of substitution observed in the bromination of thiophene-3-carboxylic acid shown in Eq. 26.18. Draw the arenium ion intermediates for all possible positions of substitution and show that the intermediate in the observed substitution is the most stable one.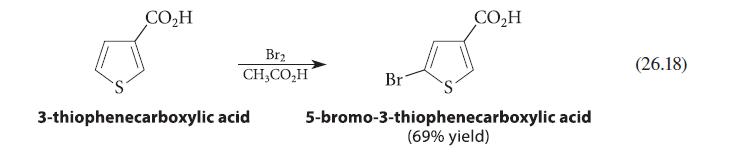
Transcribed Image Text:
CO₂H Br₂ CH,CO,H 3-thiophenecarboxylic acid Br CO₂H 5-bromo-3-thiophenecarboxylic acid (69% yield) (26.18)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The arenium ion intermediates involved in the three possible positions of substi...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Draw reaction-free energy profiles analogous to that in Fig. 16.6 in which substitution on benzene by a general electrophile E+ is compared with substitution at the para and meta positions of...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The ordinary share capital of W Ltd (which is a trading company) is owned 30% by X Ltd, 25% by Y Ltd and 45% by Z Ltd. All of these companies are UK resident and they prepare accounts to 31 March....
-
On the average, a neutron loses 63% of its energy in a collision with a hydrogen atom and 11% of its energy in a collision with a carbon atom. Calculate the number of collisions needed to reduce the...
-
Paige Company estimates that unit sales will be 10,000 in quarter 1, 14,000 in quarter 2, 15,000 in quarter 3, and 18,000 in quarter 4. Using a sales price of $70 per unit, prepare the sales budget...
-
What is the purpose of defining a role and objective for each stakeholder identified in the stakeholder analysis? AppendixLO1
-
On January 1, 2016, DIBA Company had a balance of $450,000 in its Bonds Payable account. During 2016, DIBA issued bonds with a $200,000 face value. There was no premium or discount associated with...
-
At the beginning of Year 2, the Redd Company had the following balances in its accounts: Cash Inventory Land Common stock Retained earnings $ 6,900 15,000 7,000 15,000 13,900 During Year 2, the...
-
Draw the structure of (a) 4-(dimethylamino)pyridine (b) 4-ethyl-2-nitroimidazole
-
(a) The dipole moments of pyrrole and pyrrolidine are similar in magnitude but have opposite directions. Explain, indicating the direction of the dipole moment in each compound. (b) Explain why the...
-
Total factor productivity (continuation of 13-34). Use the data given for Berkshire Corporation in Problem 13-34. Required 1. Compute Berkshire Corporations total factor productivity in 2007. 2....
-
Is there a difference between a Leader and a Manager; if yes, what and what are the differences? What is the message of the video ? https://youtu.be/TQhns5AwAkA
-
Thomas Inc. purchased 90% of Tracy Co. for $990,000 when the book value of Tracy was $1,000,000. There was no premium paid by Thomas. Tracy currently has 100,000 shares outstanding and a book value...
-
Troy Engines, Ltd., manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
A Community Hospital has two (2) service departments: Maintenance and Food Services. The hospital has three (3) patient care units, namely: General Medicine, OB, and Surgery. Additional information...
-
The following link is for the United States International Trade Commission database on current tariffs that the United States has in place: https://dataweb.usitc.gov/tariff/database This link is for...
-
What is the mass defect of the 14N nucleus?
-
Calculate the electrical conductivity of a fiber-reinforced polyethylene part that is reinforced with 20 vol % of continuous, aligned nickel fibers.
-
Complete each of the reactions given in Fig. P20.49 by giving the principal organic product(s). Give the reasons for your answers. H,C KOH PhCH Cl acid HO,C CO,H+ethylene glycolheolym) CH + Hg(OAch...
-
Give the structure of the compound C7H5O2Cl that has an IR absorption at 1685 cm-1 as well as a strong, broad O-H absorption, and the following proton NMR spectrum: 7.56 (2H, leaning d, J = 10 Hz); ...
-
Give a curved-arrow mechanism for each of the reactions given in Fig. P20.51. OC,H, dil. HCI (catal ,- OC2Hs an orthoester 0 CIH O C CH dil HOI (catalyst) CH OH (e) H,C ,--, carbon monoxide CH Ph Ph...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


