(a) The dipole moments of pyrrole and pyrrolidine are similar in magnitude but have opposite directions. Explain,...
Question:
(a) The dipole moments of pyrrole and pyrrolidine are similar in magnitude but have opposite directions. Explain, indicating the direction of the dipole moment in each compound.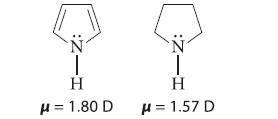
(b) Explain why the dipole moments of furan and pyrrole have opposite directions.
(c) Should the dipole moment of 3,4-dichloropyrrole be greater than or less than that of pyrrole? Explain.
Transcribed Image Text:
-Z: H H = 1.80 D -Z: T H H = 1.57 D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Because nitrogen is an electronegative atom the CN bond dipoles in pyrrolidine are directed towards the nitrogen and their resultant is also directe...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The dipole moments of pyrrole and pynolidine are similar in magnitude but have opposite directions. Explain, indicating the direction of the dipole moment in each compound. Should the dipole moment...
-
The compounds FCl and ICl have dipole moments that are similar in magnitude (0.9 and 0.7 D, respectively) but opposite in direction. In one compound, chlorine is the positive end of the dipole; in...
-
The dipole moments of furan and tetrahydrofuran are in the same direction. One compound has a dipole moment of 0.70 D, and the other has a dipole moment of 1.73 D. Which is which?
-
A close company which prepares accounts to 31 March each year is owned and managed by a single shareholder/director who is not a Scottish taxpayer and who is paid a salary of 5,000 per month. In...
-
(a) Use the result of part (e) of Problem 72 (Equation 40-23) to show that after N head-on collisions of a neutron with carbon nuclei at rest, the energy of the neutron is approximately (0.714)NE0,...
-
When you changed the value of a deposit in the Mailing List table, which of the following was true? The formula had to be reformatted The formula had to be recreated The formula updated automatically...
-
Why should a project manager be concerned with monitoring a projects progress? AppendixLO1
-
Harold, Jasmine, Caesar, and Yuan form Microhard.com, LLC, a limited liability company, to sell computer hardware and software over the Internet. Microhard.com, LLC, hires Heather, a recent graduate...
-
Use the following information for the next four (4) questions: The following summarizes the capital transactions of partners Aida and Dina which started operation during 202X Investments Drawings)...
-
Explain the position of substitution observed in the bromination of thiophene-3-carboxylic acid shown in Eq. 26.18. Draw the arenium ion intermediates for all possible positions of substitution and...
-
Imidazole is a base; the pK a of its conjugate acid is 6.95. On which nitrogen does imidazole protonate?
-
Choose the letter of the correct setup to perform synthetic division on the indicated quotient. x 3x + 2 x-1 A. 11 -3 2 B. -11 -3 2 C. 1)1 -3 02 D. 1)-1 3 0 -2
-
Saskatchewan Soy Products (SSP) buys soy beans and processes them into other soy products. Each tonne of soy beans that SSP purchases for $300 can be converted for an additional $200 into 500 lbs of...
-
Pharoah Acres sponsors a defined-benefit pension plan. The corporation's actuary provides the following information about the plan: January 1, 2025 December 31, 2025 Vested benefit obligation $510...
-
Company panther is compelled to pick between two machines An and B. The two machines are planned in an unexpected way, yet have indistinguishable limit and do the very same work. Machine A costs...
-
On April 30, 2023, a company issued $600,000 worth of 5% bonds at par. The term of the bonds is 9 years, with interest payable semi- annually on October 31 and April 30. The year-end of the company...
-
Identify the following; MethodBodyReturn statementReturn typeParameter Look at this example we saw in our Methods lesson: public double findTheArea (double length, double w idth) { double area =...
-
Calculate the binding energy per nucleon of the 3115P nucleus.
-
Modify the CYK algorithm so that it applies to any CFG, not just those in CNF.
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
Give the structure for an isomer of compound A that has a melting point of 208C and NMR spectra that are almost identical to those of A.
-
Provide a structure for each of the following compounds. C 9 H 10 O 3 : IR 2400-3200, 1700, 1630 cm 1 NMR: 1.53 (3H, t, J = 8 Hz); 4.32 (2H, q, J = 8 Hz); 7.08, 8.13 (4H, pair of leaning...
-
ABC company makes turbo-encabulators, customized to satisfy each customers order. They split overhead into five pools, each with its own activity driver (direct labor for manufacturing, direct labor...
-
Variable manufacturing overhead becomes part of a unit's cost when variable costing is used.Group of answer choicesTrueFalse
-
Santa Fe Corporation has computed the following unit costs for the year just ended:Direct Material used $23Direct Labor $18Fixed selling and administrative cost $18Variable manufacturing overhead...

Study smarter with the SolutionInn App


