Fischer also carried out the following pair of conversions. Again, no bonds to the asymmetric carbon were
Question:
Fischer also carried out the following pair of conversions. Again, no bonds to the asymmetric carbon were broken. Explain why this pair of conversions (but not either one alone) and the associated optical activities rule out pyramidal geometry at the asymmetric carbon, but are consistent with tetrahedral geometry.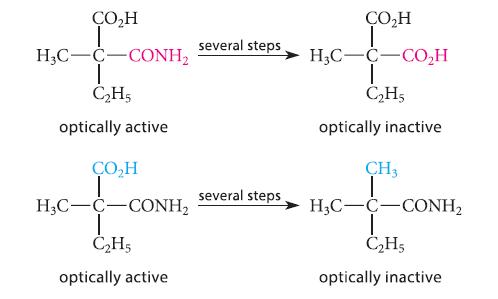
Transcribed Image Text:
CO₂H H3C-C CONH₂ T C₂H5 optically active ÇO₂H T H3C-C-CONH₂ C₂H5 optically active several steps several steps ÇO₂H T HỌC—C−CO,H | C₂H5 optically inactive CH3 HỌC–C–CONH, Ī C₂H5 optically inactive
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Neither result taken alone rules out pyramidal geometry However for an atom with two identical group...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In 1914, the chemist Emil Fischer carried out the following conversion in which optically active starting material wastransformed into a product with an identical melting point and an optical...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
VSEPR (valence state electron pair repulsion) theory was formulated to anticipate the local geometry about an atom in a molecule (see discussion in Section 25.1). All that is required is the number...
-
Utility is a type of function that occurs in eco-nomics. When a consumer receives a units of a product, a certain amount of pleasure, or utility U, is gained. The following represents a typical...
-
1. In entrapment cases, has the defendant definitely committed the crime, probably committed the crime, or probably not committed the crime? 2. Arent sting operations a useful means of catching...
-
The cantilever beam ACB shown in the figure has flexural rigidity EI = 2.1 x 106 k-in.2 Calculate the downward deflections δC and δB at points C and B, respectively, due to...
-
What are the two basic questions in inventory management discussed in the text? LO.1
-
Baylor University sold 10,000 season football tickets at $80 each for its five-game home schedule. What entries should be made (a) When the tickets were sold, and (b) After each game?
-
Morganton Company makes one product, and has provided the following information to help prepare the master budget for its first four months of operations: a. The budgeted selling price per unit is...
-
Make a model of chair cyclohexane corresponding to the leftmost model in Fig. 7.4. Raise carbon-4 so that carbons 25 lie in a common plane. This is the half-chair conformation of cyclohexane, and it...
-
Two stereoisomers of the compound (H 3 N) 2 Pt(Cl) 2 with different physical properties are known. Show that this fact makes it possible to choose between the tetrahedral and square planar...
-
On January 1, 2020, Animation Ltd., which uses ASPE, sold a truck to Letourneau Finance Corp. for $65,000 and immediately leased it back. The truck was carried on Animations books at $53,000, net of...
-
Figure < 4 ft/s 45 0.75 ft 3 ft/s 1.50 ft 1 of 1 < Part A Determine the velocity of point A on the rim of the gear at the instant shown.(Figure 1) Enter the x and y components of the velocity...
-
what ways can leaders facilitate cognitive reframing and emotional regulation techniques to promote constructive conflict resolution ?
-
What is the level of sales needed to achieve a 10% return on an investment of $10,000,000 for a restaurant (the restaurant has main products it sells: food, beverage and gift shop items) and cover...
-
1. An online computer assembling mobile phone Application provides interfaces for end users to assemble computers by selecting computer accessories with different configurations from different...
-
1. (# 3.21, Text) Plot the longitudinal and transverse coefficients of thermal expansion for a unidirectional glass-polyester composite as functions of fiber volume fraction. Assume the following...
-
Describe the locations of the bursae associated with the knee joint.
-
Name some of the various types of financial intermediaries described in the chapter and indicate the primary reason(s) each was created.
-
Assume that you have two unlabeled bottles, one of the following which contains phenol (pKa = 9.9) and one of which contains acetic acid (pKa = 4.76). In light of your answer to Problem 2.51, suggest...
-
Identify the acids and bases in the followingreactions: CH2 (a) CH3 + Ht TICIA () + TICI, CH "CH (c) + NaH +. Na+ 2 (d) +
-
Which of the following pairs represent resonance structures? (a) CH3C=N-O: and CH3C=N-o: (b) :0: :C C0: and (d) (c) : and :CH2-N CH2=N NH3 and NH2
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


