From what epoxide and what nucleophile could each of the following compounds be prepared? (Assume each is
Question:
From what epoxide and what nucleophile could each of the following compounds be prepared? (Assume each is racemic.)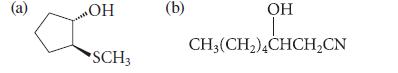
Transcribed Image Text:
(a) ОН SCH3 (b) OH CH3(CH₂)4CHCH₂CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The strategy in this problem is to let the OH group originate from the epo...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. How could each of the following compounds be prepared from a hydrocarbon in a single step? 1. 2. 3. b. What other organic compound would be obtained from each synthesis? OH Br Br CH CH HO
-
From what epoxide and what nucleophile colld each of the following compound be prepared Inppued? (Assums each is racemic.) C,H OH/H,O CH CH2 sodium azide
-
From which alkyne could each of the following compounds be prepared by acid- catalyzed hydration? (a) (c) CH3CCHCHCH3 (b) O (CH3)3C-C-CH3
-
1. Describe briefly how computers work and become tools for committing cyber-related crimes. 2. Use of proxies and VPNs is one of the methods cybercriminals try to cover their digital track and...
-
John Verner is the controller for BioMedic, Inc., a biotechnology company. John is finishing his preparation of the preliminary financial statements for a meeting of the board of directors scheduled...
-
Illinois Steel is a specialty steel manufacturer that does business in the United States, Canada, and Brazil. Illinois Steel is organized as follows. The parent, Illinois Steel, is incorporated in...
-
2-7. Explain the four market-product strategies in diversification analysis.
-
On July 1, Job 88 had a beginning balance of $710. During July, prime costs added to the job totaled $640. Of that amount, direct materials were three times as much as direct labor. The ending...
-
If it costs $300/SF to develop a new class A office building and the annual net rent for office space is $22.50/SF, what is the cap rate that would make additional development feasible? Also, if...
-
Explain each of the following facts with a mechanistic argument. (a) When butyl methyl ether (1-methoxybutane) is treated with HI and heat, the initially formed products are mainly methyl iodide and...
-
The chlorohydrin trans-2-chlorocyclohexanol reacts rapidly in base to form an epoxide. The cis stereoisomer, however, is relatively unreactive and does not give an epoxide. Explain why the two...
-
Suppose the solution of the boundary-value problem y'' + Py' + Qy = f(x), y(a) = 0, y(b) = 0, a < b, is given by y p (x) = b a G(x,t)f(t)dt where y 1 (x) and y 2 (x) are solutions of the associated...
-
Compared to other majornations, the United States spends________ on health care and achieves________ efficiency. A. more; about the same B. about thesame; less C. more; less D. less; less E. less;...
-
Studying other cultures through a humanistic lens allows people to understand how different cultures came about and how and why people behave differently from one place to another (Lombrozo, 2015)....
-
4. Assume that G and T are exogenous, and C is determined by the standard. consumption function, but that investment is now endogenous and responds to income: I = b + bY. Assume c + b < 1. (a)...
-
4. You have decided it's time to buy a house, and you have found the one you want. The price is $500,000, and you will pay 10% in cash and will take a mortgage on the balance. The annual interest...
-
Differentiate. G(x) = (2x+3) (9x+ (x) G'(x)=
-
Derive the formula A = ½ r2t for the area of a sector of a circle. Here r is the radius and t is the radian measure of the central angle (see Figure 17).
-
In the busy port of Chennai, India, the number of containers loaded onto ships during a 15-week period is as follows: 1. Develop a linear trend equation to forecast container loadings. 2. Using the...
-
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material for the biosynthesis of mevalonate, as shown in figure. At what positions in mevalonate...
-
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material and that the mevalonate pathway is followed. Identify the positions in ?-cadinol where...
-
Assume that acetyl CoA containing a 14C isotopic label in the carboxyl carbon atom is used as starting material and that the mevalonate pathway is followed. Identify the positions in squalene where...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


