Give a curved-arrow mechanism for the reaction in Eq. 22.34. Your mechanism should show why two equivalents
Question:
Give a curved-arrow mechanism for the reaction in Eq. 22.34. Your mechanism should show why two equivalents of NaOH must be used.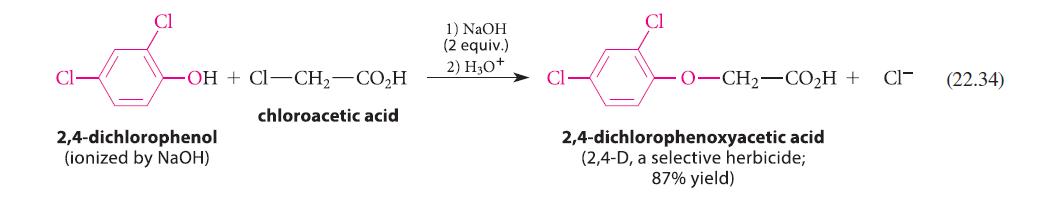
Transcribed Image Text:
-OH+CI-CH₂-CO₂H chloroacetic acid 2,4-dichlorophenol (ionized by NaOH) 1) NaOH (2 equiv.) 2) H3O+ Cl O–CH2–COH + 2,4-dichlorophenoxyacetic acid (2,4-D, a selective herbicide; 87% yield) CI- (22.34)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
One equivalent of NaOH is consumed by its reaction with the carb...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
2. Draw the curved arrow mechanism for the reaction between 2-methoxyphenoxide and 3-chloro-1,2-propanediol, in the guaifenesin synthesis. Be sure to clearly show the bimolecular transition state.
-
Give a curved-arrow mechanism for the reaction in Eq. 18.85. Be sure to identify the electrophilic species in the reaction and to show how it is formed. Eq. 18.85 CH3 CH 70% H,504 80 C OH + H2O CH3...
-
Exercise: derive the following relation. C = v v Is = T = T --(G)-(G)-(G), = - = Cp Co = T = -T 2 Iv (0) (0) ---(0), (0%), v T
-
Two positive charges, each with charge q = 2.5 nC, are placed as shown in the diagram. The distance d 0.42 m. Find the net electric potential at the point x = 0, y = 0.12 m.
-
Dobbs Wholesale Antiques makes all sales under terms of FOB shipping point. The company usually ships inventory to customers approximately one week after receiving the order. For orders received late...
-
Farmers in a valley are subject to occasional flooding when heavy rains cause the river to overflow. They have asked the federal government to build a dam upstream to prevent flooding. The...
-
The United States and Russia belong to an economic bloc, how is this the case? LO.1
-
Interstate 81 through southwest Virginia is heavily traveled by long-distance truckers. To cut down on accidents, The Virginia State Patrol carries out random inspections of a trucks weight and the...
-
Camille Sikorski was divorced in 2018. She currently provides a home for her 15-year-old daughter Kaly. Kaly lived in Camilles home for the entire year, and Camille paid for all the costs of...
-
Give the structure of a compound C 6 H 10 O 2 that gives succinic acid and iodoform on treatment with a solution of I 2 in aqueous NaOH, followed by acidification.
-
Explain why: (a) The rate of iodination of optically active 1-phenyl-2-methyl-1-butanone in acetic acid/HNO 3 is identical to its rate of racemization under the same conditions. (b) The rates of...
-
In Exercises 1124, find the limit and discuss the continuity of the function. lim (x, y) (3, 1) (x-2y)
-
Below are listed some additional common performance measures not listed in Exhibit 2.1. Which type of employee (senior managers, middle managers, or frontline operations managers) would typically use...
-
If you have a steam distillation system with immiscible organic and water phases plus a vapor phase, two volatile organic compounds plus a nonvolatile organic compound, at equilibrium how many...
-
An auditor is using difference estimation for the confirmation of accounts receivable in the audit of Lafferty Hardware Supply. A random sample of 100 positive confirmations has been sent to...
-
Canterbury Convenience Stores (CCS) is a newly formed organization in Christchurch, New Zealand. It comprises 10 moderately sized convenience stores that previously operated independently of each...
-
Orchard Distributions Pte. Ltd. is a large, Singaporean-based distributor of clothing products to other companies throughout Southeast Asia. Orders are received from customers either by telephone,...
-
True or false: uAu
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
Write a detailed mechanism for the following reaction. HBr (excess) 2 Br
-
Provide a mechanism for the following reaction. HCI CI
-
Propose structures for each of the following products derived from oxirane (ethylene oxide): (a) (b) (c) (d) (e) Methyl Cellosolve cat. HA, CH1002 EtOH Ethyl Cellosolve H2O
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


