Give the principal product(s) expected when 1-hexyne or the other compounds indicated are treated with each of
Question:
Give the principal product(s) expected when 1-hexyne or the other compounds indicated are treated with each of the following reagents: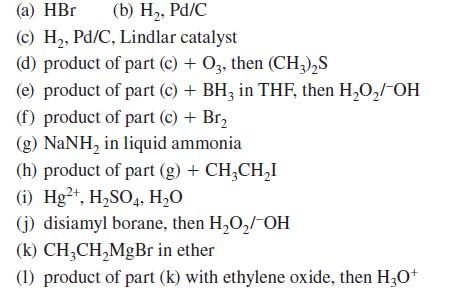
Transcribed Image Text:
(a) HBr (b) H₂, Pd/C (c) H₂, Pd/C, Lindlar catalyst (d) product of part (c) + O3, then (CH3)₂S (e) product of part (c) + BH3 in THF, then H₂O₂/-OH (f) product of part (c) + Br₂ (g) NaNH, in liquid ammonia (h) product of part (g) + CH3CH₂I (i) Hg2+, H₂SO4, H₂O (j) disiamyl borane, then H₂O₂/-OH (k) CH3CH₂MgBr in ether (1) product of part (k) with ethylene oxide, then H3O+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a e h k CH3CHCHCHCCH Br CHCHCHCHCHCHOH b C...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the principal products expected when 4-octyne or the other compounds indicated are treated with each of the following reagents: (a) H2, Pd/C catalyst (b) H2, Lindlar catalyst (c) Product of (b)...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
What is the benefit to a company from a securities underwriter? They study the market and advise companies on where to set their IPO share price. They help companies to reduce the risk associated...
-
In 2005, after years of positive growth in the housing market, sales and prices began to slow down and then decline. By 2007 many large home-construction companies were reporting net losses. The data...
-
The accompanying data set consists of observations on shear strength (lb) of ultrasonic spot welds made on a certain type of alclad sheet. Construct a relative frequency histogram based on ten equal...
-
For constants > 0 and > 0, assume Mt def = et Ct C0 is an SDF process, where C denotes aggregate consumption. Assume that dC C = dt + dB (13.56) for stochastic processes and . (a) Apply Its...
-
1. Which promotional mix elements does Snickers use? 2. How does this Snickers campaign demonstrate the characteristics of integration? 3. What grade would you give Snickers on integration...
-
Please answer only QUESTION #3 in the context of Apple In. (in details) The second picture shows all the necessary info needed. (cost of equity, return on Applw etc.) *rAAPL ( return of Apple) *rm...
-
A box labeled C 6 H 10 isomers contains samples of three compounds: A, B, and C. Along with the compounds are the IR spectra of A and B, shown in Fig. P14.36. Fragmentary data in a laboratory...
-
The following compound is an intermediate in one synthesis of the mating pheromone of the female Indian meal moth. Show how this compound can be converted into the pheromone in a single reaction. H...
-
FUTURE VALUE It is now January 1, 2014. Today you will deposit $1,000 into a savings account that pays 8%. a. If the bank compounds interest annually, how much will you have in your account on...
-
The following information summarizes the activities in the Mixing Department for the month of March. Beginning inventory 1 , 0 0 0 units, 8 0 % complete Started and completed 2 4 , 5 0 0 units Ending...
-
What is your recommendation for the maximum size of coarse aggregate for the following situation? A continuously reinforced concrete pavement cross section contains a layer of No. 6 reinforced bars...
-
On January 1, 2024, Winn Heat Transfer leased office space under a three-year operating lease agreement. The arrangement specified three annual lease payments of $72,000 each, beginning December 31,...
-
A closed square pyramid tank (base width: 6.0 m; height 3.0 m), sitting on its square base, has a 1.0 m depth of water. Suppose this tank is inverted (turned upside down) and is made to stand on its...
-
P.4.3 Apply a Taylor series expansion to a mixed backward formula for the first derivative: (Ux)i = 1 Ax (aui-2+ bui-1 + cu + dui+1) Derive the family of second order accurate formulas and the...
-
In problems 1-5, find the indicated limit or state that it does not exist. 1. 2. 3. 4. 5. r2 ma x + 2 1 1 lim
-
The percentage of completion and completed contract methods are described in the FASB ASC. Search the codification to find the paragraphs covering these topics, cite them, and copy the results.
-
1, 3-Cyclopentadiene is very reactive in DielsAlder cyclo addition reactions, but 1, 3-cyclohexadiene is less reactive and 1, 3-cycloheptadiene is nearly inert. Explain. (Molecular models are...
-
1, 3-Pentadiene is much more reactive in Diels?Alder reactions than 2, 4-pentadienal. Why might this be? H 1,3-Pentadiene 2,4-Pentadienal
-
How could you use Diels?Alder reactions to prepare the following products? Show the starting diene and dienophile in each case. (a) (b) CN C (c) (d) H.
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


