Give the starting material required for the synthesis of each of the following compounds by a Dieckmann
Question:
Give the starting material required for the synthesis of each of the following compounds by a Dieckmann condensation.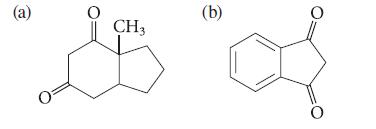
Transcribed Image Text:
(a) CH3 (b) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a b EtO CH3 CH3 or of OEt CH3 C HC OEt CH3 Either answer is sati...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline a synthesis for each of the following compounds from the indicated starting material and any other reagents. (a) l-chloro-3, 5-dinitrobenzene from benzene (b) 2-chloro-4,6-dinitrophenol from...
-
What starting materials could be used to synthesize each of the following compounds by a Sonogashira coupling reaction? (a) (b) CI
-
Starting with benzene or toluene, outline a synthesis of each of the following compounds using diazonium salts as intermediates. (You need not repeat syntheses carried out in earlier parts of this...
-
First United Bank Inc. is evaluating three capital investment projects by using the net present value method. Relevant data related to the projects are summarized as follows: Instructions 1. Assuming...
-
Use the Mountain Cycles data in Short Exercise 6-5. Requirements 1. Journalize the August 16 purchase of inventory on account. 2. Journalize the August 31 sale of inventory on account. Mountain sold...
-
When may reference to other agreements be made in a negotiable instrument without destroying its negotiability?
-
Identify and assess the main investment opportunities in Qatar for overseas investors? LO.1
-
Imperial Carpet has the following unadjusted trial balance as of March 31, 2012. The debit and credit totals are not equal as a result of the following errors:a. The balance of cash was understated...
-
View O Help Search Table Design Layout E 21 AaBbcDa AaBbceDi AaBbCcDd AaBbCD AaBbco Emphasis 1 Heading 1 1 Normal Strong Subtitle Paragraph Styles Part 1-45 Marks Como Corporation manufactures car...
-
Suppose a sample of acetyl-CoA labeled at the carbonyl carbon with the radioisotope 14 C is introduced into the fatty-acid synthase system, and palmitic acid (the 16-carbon fatty acid; Eq. 22.76) is...
-
Hydroxide ion is about as basic as ethoxide ion. Would NaOH be a suitable base for the Claisen condensation of ethyl acetate? Explain by writing suitable equations.
-
The cube in FIGURE EX24.8 contains no net charge. The electric field is constant over each face of the cube. Does the missing electric field vector on the front face point in or out? What is the...
-
Refer to Figure 11.2: Is it more costly to build in Los Angeles or in Washington DC? What is the cost difference? Figure 11.2 Location Factors Costs shown in RSMeans Square Foot Costs are based on...
-
Suppose the prism in Figure P33.27 is immersed in a liquid in which the speed of light is lower than the speed of light in glass. Describe what happens to the light shown entering at normal...
-
Each year, the AICPA issues a general audit risk alert document and a number of industry audit risk alerts. If you can obtain access to a current copy of either the general alert or one of the...
-
The multieffect distillation system shown in Figure 11-4 appears to be able to cut energy use in half; however, the reduction is not this large. Explain why. Figure 11-4 F PL D, D Reflux B PH
-
Schemes 11-6E and 11-6F accomplish the same task of removing and purifying an intermediate component. a. What factors enter into the decision to use scheme \(11-6 \mathrm{~F}\) instead of \(11-6...
-
(a) Show that the matrix has λ = ± i as incomplete complex conjugate eigenvalues. (c) Explain the behavior of a typical solution. Why is the zero solution not stable? 0110 1001...
-
What mass of KBr (in grams) should you use to make 350.0 mL of a 1.30 M KBr solution?
-
In addition to more highly chlorinated products, chlorination of butane yields a mixture of compounds with the formula C4H9Cl. (a) Taking stereochemistry into account, how many different isomers with...
-
Chlorination of (R)-2-chlorobutane yields a mixture of dichloro isomers. (a) Taking into account stereochemistry, how many different isomers would you expect? Write their structures. (b) How many...
-
Peroxides are often used to initiate radical chain reactions such as in the following radical halogenation. (a) Using bond dissociation energies in Table 10.1, explain why peroxides are especially...
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


