Suppose a sample of acetyl-CoA labeled at the carbonyl carbon with the radioisotope 14 C is introduced
Question:
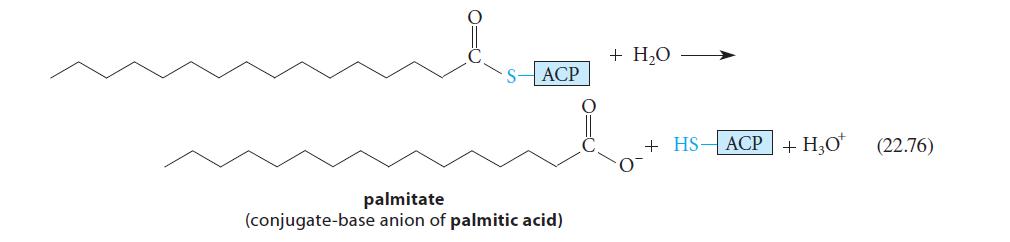
Suppose a sample of acetyl-CoA labeled at the carbonyl carbon with the radioisotope 14C is introduced into the fatty-acid synthase system, and palmitic acid (the 16-carbon fatty acid; Eq. 22.76) is isolated. Which carbons of palmitic acid should be radiolabeled?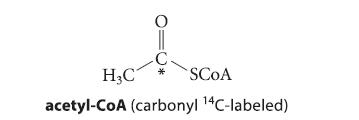
Transcribed Image Text:
O į H₂C acetyl-CoA (carbonyl 14C-labeled) SCOA
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Acetyl CoA is the ultimate source of carbon atoms in fatty acid biosynt...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Because the radioactive isotope carbon-14 is used at very low (tracer) levels, its presence cannot be detected by spectroscopy. It is generally detected by counting its radioactive decay in a device...
-
The CO2 produced in one round of the citric acid cycle does not originate in the acetyl carbons that entered that round. If acetyl CoA is labeled with 14C at its carbonyl carbon, how many rounds of...
-
3. How should a temperature change affect the potential of a standard cell, or a cell in which Q=1? a. it should increase b. it should decrease c. it should stay the same 4. What happened to the...
-
Dozier Industries Inc. manufactures only one product. For the year ended December 31, 2014, the contribution margin increased by $38,500 from the planned level of $1,386,000. The president of Dozier...
-
Refer to Short Exercises 6-3 through 6-8. After completing those exercises, answer the following questions: Requirements 1. Which method of inventory accounting produced the lowest cost of goods...
-
What does the interpretation of the law in this case suggest to business persons who sell products labeled with statements mandated by federal or state law?
-
What are the major exports into Qatar and are these likely to change? LO.1
-
Comparative statement of financial position accounts of Jensen Limited, which follows IFRS, appear below: Data from Jensen's 2017 income statement follow: Additional information: 1. Equipment that...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 86,000 Daks each year at a selling price of $60 per unit. The companys unit costs at this level of activity...
-
Using abbreviated structures like the ones used in this section, outline the steps that convert hexanoyl-ACP into octanoyl-ACP during fatty-acid biosynthesis.
-
Give the starting material required for the synthesis of each of the following compounds by a Dieckmann condensation. (a) CH3 (b) 0
-
Under which conditions would a plant manager elect to use a fixedorder quantity model as opposed to a fixedtime period model? What are the disadvantages of using a fixedtime period ordering system?...
-
Gordon Rivers, the city manager of Saratoga, Florida, pitched the proposed design schedule back at Jay Andrews. Jay Andrews is the project manager for Major Design Corporation (MDC). The city of...
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts straight to retained earnings. The balance of \(\$ 8,500\) in the retained earnings account...
-
Draw a Keynesian cross diagram to show the effects of a rise in autonomous expenditure on an economy operating below full employment output.
-
Governments in many countries are acutely aware of the environmental problems that vehicle emissions can have. Many car manufacturers are exploring the production of electric vehicles, but production...
-
Draw a simple diagram of John Woodens pyramid of success. You can find it at the official Wooden website www.coachwooden.com/index2.html.
-
Let A be a real 3 x 3 matrix, and assume that the linear system has a periodic solution of period P. Prove that every periodic solution of the system has period P. What other types of solutions can...
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
Suggest a method for separating and isolating the CH3Cl, CH2Cl2, CHCl3, and CCl4 that may be formed as a mixture when methane is chlorinated. (You may want to consult a handbook.) What analytical...
-
Which of the following compounds can be prepared by radical halogenation with little complication by formation of isomeric by-products? CI CI
-
The radical reaction of propane with chlorine yields (in addition to more highly halogenated compounds)1-chloropropane and 2-chloropropane. Cl
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


