Give the structure of an aldehyde C 8 H 8 O 2 that would be oxidized to
Question:
Give the structure of an aldehyde C8H8O2 that would be oxidized to terephthalic acid by KMnO4.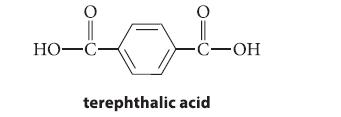
Transcribed Image Text:
HO-C- O -C-OH terephthalic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The starting material has five degrees of ...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure of an intramoleczlar substitution product and an intermolecular substitution product that might be obtained from 4-bromo-l-butanol on treatment with one equivalent of NaOH. Which...
-
Give the structure of the product derived by intramolecular aldol condensation of the keto aldehyde shown: O CHI KOH. H-0 CH CCH CCHOCHIoO CH3
-
Give the structure of the product derived by intramolecular aldol condensation of the keto aldehyde shown:
-
According to a study by the American Pet Food Dealers Association, 63% of U.S. house-holds own pets. A report is being prepared for an editorial in the San Francisco Chronicle. As a part of the...
-
XM, Ltd., was a small engineering firm that built hi-tech robotic devices for electronics manufacturers. One very complex device was partially completed at the end of 2014. Barb McLauren, head...
-
Listed below are events that affect stockholders' equity. a. Reported net income of $85,000. b. Paid a cash dividend of $10,000. c. Reported sales revenue of $120,000. d. Issued common stock of...
-
Web-based exercise. Tables of areas under a Normal curve, like Table B at the back of this book, are still common but are also giving way to applets that let you find areas visually. Go to the...
-
Using optimization theory, analyze the following quotations: a. The optimal number of traffic deaths in the United States is zero. b. Any pollution is too much pollution. c. We cannot pull U.S....
-
292 Chapter 8 Compound Interest: Future Value and Present Value CONCEPT QUESTIONS most preferred"): 6% per mpound rate of return. How 1. For a six-month investment, rank the following interest rates...
-
Give the products expected (if any) when acetone reacts with each of the following reagents. (a) HO+ (b) NaBH4 in CHOH, then HO (c) CrO3, pyridine (d) NaCN, pH 10, HO (e) CH3OH (excess), HSO4 (trace)...
-
Outline a Wittig synthesis for each of the following alkenes; give two Wittig syntheses of the compound in part (a). CH0- -CH=CH- (mixture of cis and trans) (b) CH3 I HC=CCHCH3 CHCH=
-
If a project breaks even on a financial basis, what do you know about its discounted payback?
-
Business meeting simulation: Start with preparing an email and send it to all the participant of the meeting to inform about the meeting topic/ agenda/time of the meeting/ room #. Create a meeting...
-
Topic #1: Rayleigh-Ritz Method (RRM) Problem 1 a) Find the exact solution for the beam torsion problem shown above. The loading consists of a uniformly distributed torque m and a point torque M...
-
Assume that a $10,000, five-year, 8% term note, is issued on October 1, 20X3: what is the Journal Entry Cash Note Payable 10,000 10,000 Cash 10,000 Accounts Payable 10,000 Note Payable 10,000 Cash...
-
How do I get help with combining a final paper over a period of 6 weeks for my Senior Management Seminar course MGMT-495 at American Public University.
-
Question: Fillmore, Inc. specializes in customized optimization spreadsheet software. The results of the company's operations during the prior year ( 2 0 ?are given in the following table. All...
-
(a) Prove that every positive definite matrix K has a unique positive definite square root, i.e., a matrix B > 0 satisfying B2 = K. (b) Find the positive definite square roots of the following...
-
Given the table below, about how much force does the rocket engine exert on the 4.0 kg payload? Distance traveled with rocket engine firing (m) Payload final velocity (m/s) 500 320 490 310 1020 450...
-
Diacyl peroxides decompose readily when heated. (a) What factor accounts for this instability? (b) The decomposition of a diacyl peroxide produces CO2. How is it formed? (c) Diacyl peroxides are...
-
Write a structural formula for each of the following compounds: (a) Octanoic acid (b) Propanamide (c) N, N-Diethylhexanamide (d) 2-Methyl-4-hexenoic acid (e) Butanedioic acid (f) 1, 2-Benzenedioic...
-
Give an IUPAC systematic or common name for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CH3CN Cl NH2
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


