Outline a Wittig synthesis for each of the following alkenes; give two Wittig syntheses of the compound
Question:
Outline a Wittig synthesis for each of the following alkenes; give two Wittig syntheses of the compound in part (a).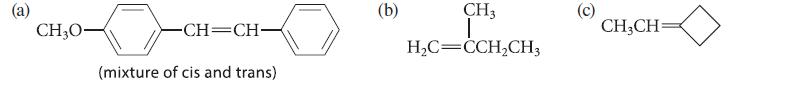
Transcribed Image Text:
CH₂0- -CH=CH- (mixture of cis and trans) (b) CH3 I H₂C=CCH₂CH3 CH₂CH=
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Either half of the alkene can in principle be derived ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Outline practical syntheses of each of the following compounds from alcohols containing no more than four carbon atoms and any necessary organic or inorganic reagents. In many cases the desired...
-
Outline practical syntheses of each of the following compounds from alcohols containing no more than four carbon atoms and any necessary organic or inorganic reagents. In many cases the desired...
-
Starting with an appropriate alkyl halide and base, outline syntheses that would yield each of the following alkenes as the major (or only) product: (a) (b) (c) (d) (e)
-
The dehydration butanol of alumina is carried out over a silica-alumina catalyst at 680K. CH3CH2CH2CH20H------->cat CH3CH=CHCH3 + H2O The rate law is -r Bu = KPBU/(1+KBuPBul with k= 0.054...
-
Amazon.comlike all other businessesmakes adjusting entries prior to year-end in order to measure assets, liabilities, revenues, and expenses properly. Examine Amazons balance sheet and Note 3. Pay...
-
1. At December 31, Pitt Inc. has assets of $12,900 and liabilities of $6,300. What is the stockholders' equity for Pitt at December 31? a. $6,600 b. $6,300 c. $18,100 d. $19,200 Use the following...
-
Web-based exercise. If you did any of Exercises 13.21 to 13.30, check your calculations using the Normal Curve applet described in the previous exercise.
-
Redraft the following issues in the format presented in this chapter. Part A Can a bystander who witnesses the death of a victim from three blocks away recover for negligent infliction of emotional...
-
Explain how you expect this course Undergrad accounting Capstone will help you move forward in your current or future career. Your friend made this comment: "My major is biology and I plan to do...
-
Give the structure of an aldehyde C 8 H 8 O 2 that would be oxidized to terephthalic acid by KMnO 4 . HO-C- O -C-OH terephthalic acid
-
Outline a synthesis of 1,4-dimethoxy-2-propylbenzene from hydroquinone (p-hydroxyphenol) and any other reagents.
-
Which type of organization design best fits an uncertain and changing environment? (a) Mechanistic (b) Bureaucratic (c) Organic (d) Traditional
-
Your client who is currently using only Facebook and Instagram wants to try LinkedIn. What would you do first ? and why ?
-
Exro Technologies Corp. (Exro) is an upstart Canadian company that specializes in the manufacture of electric motors and batteries for electric bikes. Exro uses a job costing system and during...
-
How do neurobiological mechanisms, such as the stress response system and emotional regulation pathways, influence the dynamics of conflict escalation and resolution?
-
What is the consideration to invest in the company which has recorded revenue growth over the past 5 years but the Debt to Equity ratio also increase significantly to 5.72?
-
Builder Products, Incorporated, uses the weighted-average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....
-
Prove that A = AT has a repeated eigenvalue if and only if it commutes, AJ = JA, with a nonzero skew-symmetric matrix: JT = - J 0.
-
Inexhaustible collections of ONPOs are not required to be capitalized or depreciated, if certain criteria are met. Why is this so, and what accounting and reporting recognition, if any, is required...
-
Experiments show that the molecular weight of acetic acid in the vapor state (just above its boiling point) is approximately 120. Explain the discrepancy between this experimental value and the true...
-
Amides are weaker bases than corresponding amines. For example, most water-insoluble amines (RNH2) will dissolve in dilute aqueous acids (aqueous HCl, H2SO4, etc.) by forming water-soluble...
-
While amides are much less basic than amines, they are much stronger acids. Amides have pKa values in the range 14-16, whereas for amines, pKa = 33-35. (a) What factor accounts for the much greater...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


