Give the structure of an alkene that would give 2-bromopentane as the major (or sole) product of
Question:
Give the structure of an alkene that would give 2-bromopentane as the major (or sole) product of HBr addition.
(The numbers are for reference in the solution.)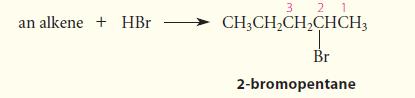
Transcribed Image Text:
an alkene + HBr 3 2 1 CH3CH₂CH₂CHCH3 Br 2-bromopentane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The bromine of the product comes from the HBr However there are many hydrogens in the product Which ...View the full answer

Answered By

Hassan Ali
I am an electrical engineer with Master in Management (Engineering). I have been teaching for more than 10years and still helping a a lot of students online and in person. In addition to that, I not only have theoretical experience but also have practical experience by working on different managerial positions in different companies. Now I am running my own company successfully which I launched in 2019. I can provide complete guidance in the following fields. System engineering management, research and lab reports, power transmission, utilisation and distribution, generators and motors, organizational behaviour, essay writing, general management, digital system design, control system, business and leadership.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following cases, provide the structure of an alkene that would give the alcohol as the major (or only) product of hydroboration--oxidation. CH CH CH CH OH CH, CH,
-
Draw the structure of (a) A five-carbon alkene that would give the same product of HBr addition whether peroxides are present or not. (b) A compound with the formula C6H12 that would not undergo...
-
In each case, give the structure of an eight-carbon alkene that would yield each of the following compounds (and no others) after treatment with ozone followed by dimethyl sulfide. (b)...
-
Debug the given C program to read and print N array elements. Find 10 errors in the given Code and correct the errors. (5Marks) NOTE: Copy and PASTE the correct program in the space below. /*Arrays...
-
1. What tort is at issue here? 2. What are the elements of this tort? 3. Why did the trial court dismiss the case? 4. What did the defendant do here?
-
Sulfur tetra-fluoride (SF4) reacts slowly with O2 to form sulfur tetra-fluoride monoxide (OSF4) according to the following unbalanced reaction: SF4 (g) + O2 (g) OSF4 (g) The O atom and the four F...
-
Describe the various scenarios for project termination. AppendixLO1
-
In a study of 413 nonprofits nationwide, 83 indicated that turnover has been the biggest employment challenge at their organization. (Source: Nonprofit HR, 2014 Nonprofit Employment Practices Survey,...
-
Exercise 9-18 PROBLEM 9-18 Comprehensive Variance Analysis LO9-4, LO9-5, LO9-6 Miller Toy Company manufactures a plastic swimming pool at its Westwood Plant. The plant has been experiencing problems...
-
Calculate the unsaturation number for each of the formulas in parts (a) and (b) and each of the compounds in parts (c) and (d). (a) C 3 H 4 Cl 4 (b) C 5 H 8 N 2 (c) Methylcyclohexane (d)...
-
Calculate the standard enthalpy difference between the cis and trans isomers of 2-butene. Specify which stereoisomer is more stable. The heats of formation are, for the cis isomer, 27.40 kJ mol 1 ,...
-
x 2 2xy + y 2 = 0 and y = 9/x Quantity A is greater. Quantity B is greater. The two quantities are equal. The relationship cannot be determined from the information given. Quantity A y Quantity B 3
-
Business Solutions's second-quarter 2022 fixed budget performance report for its computer furniture operations follows. The $175,750 budgeted expenses include $126,000 in variable expenses for desks...
-
Problem 2 (Numerical Integration) Using switch Statement and functions, write a single code to compute the following integral. 0 10 x +4 dx case 1: RECTANGULAR () // Rectangular rule case 2:...
-
Do you believe the elasticity of illicit narcotics is inelastic and if legalized demand will not increase? Do you also believe that many of society's social ills associated with drugs will ease not...
-
Stockstone Limited makes electric kettles that they currently sell at 13 each. The management believes that the company's equipment could currently produce up to 70,000 units of electric kettles per...
-
Jane Smith has worked for the Widgets, Weezles, and Warblers Corporation for the past 25 years. At a recent "Town Hall" meeting, Jane asked two members of the executive leadership team about their...
-
Explain why DNA replication is essential.
-
Walker, Inc., is an all-equity firm. The cost of the company's equity is currently 11.4 percent and the risk-free.rate is 3.3 percent. The company is currently considering a project that will cost...
-
Draw structures corresponding to the following IUPAC names: (a) 1, 1-Dimethylcycloocatne (b) 2-Cyclobutylhexane (c) 1, 2-Dichlorocyclopentane (d) 1, 3-Dibromo-5-methylcyclohexane
-
Name the following cycloalkanes:
-
Name the following substances, including the cis- or trans-prefix: CH2CH3 (b) , (a) H. - - CI
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


