Give the structure of an isomer of the allylic halide reactant in Eq. 17.7 that would react
Question:
Give the structure of an isomer of the allylic halide reactant in Eq. 17.7 that would react with water in an SN1 solvolysis reaction to give the same two products. Explain your reasoning.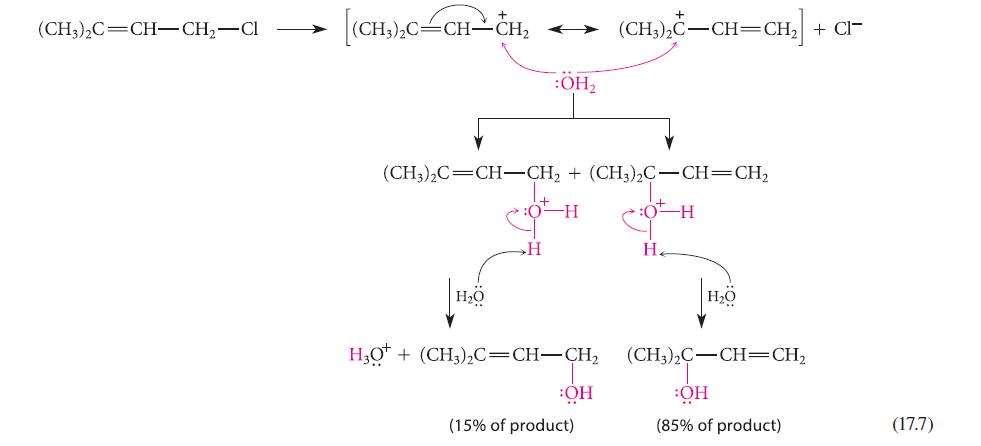
Transcribed Image Text:
(CH3)2C=CH-CH₂-Cl (CH,),C=CH–CH, H₂O :OH₂ (CH3)2C=CH-CH₂ + (CH3)2C-CH=CH₂ :0 H 0-H H HO* + (CH,),C=CH–CH, OH (15% of product) + (CH3)2C-CH=CH₂ + CI- H₂ H₂O (CH;),C–CH=CH, OH (85% of product) (17.7)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The alkyl halide is the one that reacts to give ...View the full answer

Answered By

Anum Naz
Lecturer and researcher with 10+ years of experience teaching courses in both undergraduate and postgraduate levels. Supervised 17 BA theses, 07 MA theses, and 1 Ph.D. dissertations. Edited and co-authored 2 monographs on contemporary trends in political thought. Published over articles in peer-reviewed journals.
4.80+
11+ Reviews
52+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The S N 1 solvolysis of cinnamyl chloride in water gives two structurally isomeric alcohols (neglect stereoisomers). (a) Show five resonance structures of the carbocation intermediate. In each of...
-
Give the structure of a hydrocarbon that has six carbon atoms and a. Three vinylic hydrogens and two allylic hydrogens. b. Three vinylic hydrogens and one allylic hydrogen. c. Three vinylic hydrogens...
-
The two alkenes 2,3,3-trimethyl-1-butene and 1-octene were each subjected to allylic halogenation with N-bromosuccinimide. One of these alkenes yielded a single allylic bromide, whereas the other...
-
What are the premises for successful paleostress analysis?
-
Calculating components of cash outflow from operations Refer to Exhibit 5.22, which provides items from the financial statements of Information Technologies. a. How much cash did Information...
-
A refrigerator requires 200 J of work and exhausts 600 J of heat per cycle. What is the refrigerators coefficient of performance?
-
If you roll a six-sided die six times, you will roll an even number at least once.
-
If the risk-free rate is 6% and the expected rate of return on the market portfolio is 13%, is a security with a beta of 1.25 and an expected rate of return of 16% overpriced or underpriced?
-
Svahn, AB. is a Swedish manufacturer of saling yachts. The company has assembled the information shown below that pertains to two Independent decision-making contexts The company chronically has no...
-
What product(s) are expected when each of the following compounds reacts with one equivalent of NBS in CCl 4 in the presence of light and peroxides? Explain your answers. (a) cyclohexene (b)...
-
Predict the order of relative reactivities of the compounds within each series in S N 1 solvolysis reactions, and explain your answers carefully. (a) (b) Cl T -CH- CH3 (1) CH3 of CH3 (1) -C-Cl CHO...
-
The set of all real numbers. For the following exercises, determine if the set described is finite or infinite.
-
Compared to other majornations, the United States spends________ on health care and achieves________ efficiency. A. more; about the same B. about thesame; less C. more; less D. less; less E. less;...
-
Studying other cultures through a humanistic lens allows people to understand how different cultures came about and how and why people behave differently from one place to another (Lombrozo, 2015)....
-
4. Assume that G and T are exogenous, and C is determined by the standard. consumption function, but that investment is now endogenous and responds to income: I = b + bY. Assume c + b < 1. (a)...
-
4. You have decided it's time to buy a house, and you have found the one you want. The price is $500,000, and you will pay 10% in cash and will take a mortgage on the balance. The annual interest...
-
Differentiate. G(x) = (2x+3) (9x+ (x) G'(x)=
-
In evaluating the potential future profitability of a company, how would you consider irregular income items, such as extraordinary items, discontinued operations, and prior period adjustments?
-
Rowland Textile Inc. manufactures two products: sweatshirts and T-shirts. The manufacturing process involves two activities: cutting and sewing. Expected overhead costs and cost drivers are as...
-
Give the structure of each of the following compounds. (In some cases, more than one correct answer is possible.) (a) A six-carbon alkene whose proton NMR spectrum consists of one singlet (b) A...
-
Give the structure that corresponds to each of the following molecular formulas and NMR spectra: (a) C5H10; 1.5, s (b) C2H2F3I: 3.56 (q, J = 10 Hz) (c) C6H14O: 0.91 (6H, d, 7 = 7 Hz); 1.17(6H,...
-
A compound A reacts with H2 over Pd/C to give methylcyclohexane. A colleague, A1 Keen, has deduced that the compound must be either 1-methylcyclohexene or 3-methylcyelohexene. You have been called in...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


