The S N 1 solvolysis of cinnamyl chloride in water gives two structurally isomeric alcohols (neglect stereoisomers).
Question:
The SN1 solvolysis of cinnamyl chloride in water gives two structurally isomeric alcohols (neglect stereoisomers).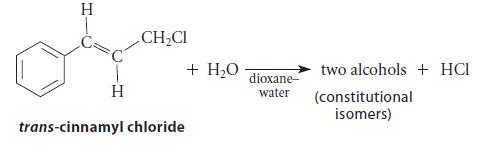
(a) Show five resonance structures of the carbocation intermediate. In each of your structures, the positive charge should be on a different carbon.
(b) Even though the positive charge is delocalized to five different carbons, only two structurally isomeric products are formed. (Neglect stereoisomers.) What are the two products? Why are more products not formed?
(c) If you have the two alcohol products but don’t know which is which, how would you use UV spectroscopy to tell them apart?
(d) A constitutional isomer A of cinnamyl chloride gives the same two products in a solvolysis reaction under the same conditions. Give the structure of A and explain.
Step by Step Answer:






