An amine R 2 NH is typically more than 20 pK a units more acidic than the
Question:
An amine R2NH is typically more than 20 pKa units more acidic than the hydrogens of the carbon analog, R2CH2 (the element effect; Sec. 3.6A). However, the acidities of 1,3-cyclopentadiene and pyrrole are an exception.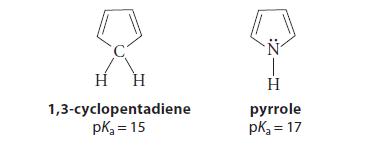
Use the theory of aromaticity to explain this exception.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





