Give the structures of the principal organic product(s) expected in each of the reactions given in Fig.
Question:
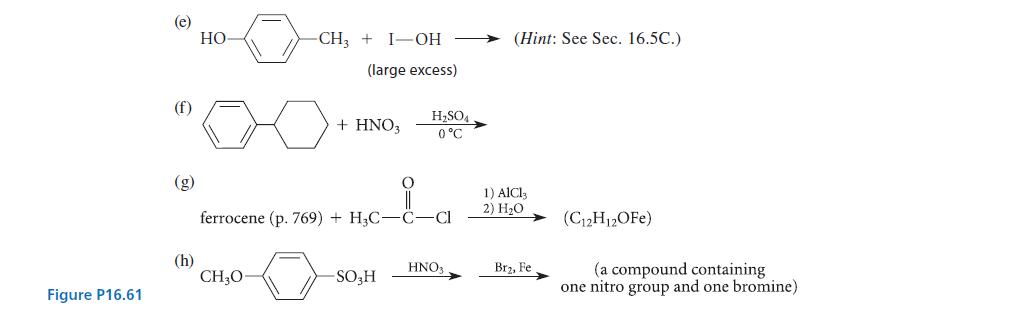
Give the structures of the principal organic product(s) expected in each of the reactions given in Fig. P16.61, and explain your reasoning.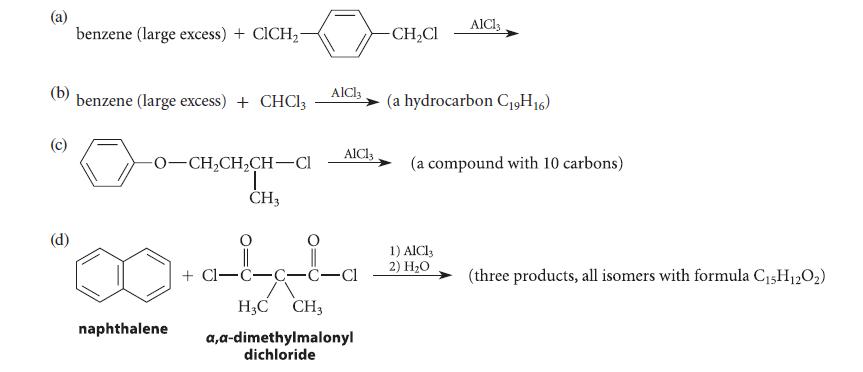
Transcribed Image Text:
(b) (d) benzene (large excess) + ClCH₂- benzene (large excess) + CHCl3 -0-CH₂CH₂CH-Cl T CH3 naphthalene AlCl3 AlCl3 +Cl-C-C-C-Cl H3C CH3 a,a-dimethylmalonyl dichloride -CH₂Cl AlCl3 (a hydrocarbon C19H16) (a compound with 10 carbons) 1) AlCl3 2) H₂O (three products, all isomers with formula C₁5H12O₂)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a Each alkyl halide group reacts with benzene in a separate FriedelCrafts alkylation reaction b The ...View the full answer

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structures of the principal organic product(s) expected in each of the reactions given in Fig. P16.59 on p. 786, and explain your reasoning. Fig. P16.59 (a) (b) (c) . ACI benzene (large...
-
Give the structures of both the reactive intermediate and the product in each of the following reactions: (a) (b) CH,CH,CCH, + HBr- CH2 CH,CH CCHH rides
-
Give the structures of the products expected when (t) valine and (2) proline (or other compounds indicated) react with each of the following reagents: (a) Ethanol (solvent). H2SO4 catalyst (b)...
-
Compare the ACA passed in 2010 with the Massachusetts health plan adopted in 2006 (use the Internet to obtain more details than we have provided in this chapter). Be sure to note the similarities and...
-
The disposal of equipment for an amount of cash greater than the carrying value of the equipment results in a cash receipt equal to the carrying value of the equipment plus the gain on the disposal,...
-
Pennies today are copper-covered zinc, but older pennies are 3.1 g of solid copper. What are the total positive charge and total negative charge in a solid copper penny that is electrically neutral?
-
Drawing one card from a standard deck of cards
-
Consider the following LP problem. MAX: 3X1 + 2X2 Subject to: 3X1 + 3X2 300 6X1 + 3X2 480 3X1 + 3X2 480 X1, X2 0 a. Sketch the feasible region for this model. b. What is the optimal solution? c....
-
HOTEL FINANCING TRENDS AND SCHEMES 173 ects. TAX ABATEMENT: An agreement with the government to forego the payment of property and other taxes. TRAINING GRANT: A subsidy offered by the government to...
-
Would 1-methoxynaphthalene nitrate more rapidly or more slowly than naphthalene at (a) Carbon-4; (b) Carbon-5; (c) Carbon-6? Explain your reasoning. 5 OCH3 N 3 1-methoxynaphthalene
-
The solvolysis reaction of 2-bromooctane in ethanol is relatively slow. However, this reaction is accelerated by the addition of silver ion (as AgNO 3 ), and one of the products is AgBr. Explain how...
-
At December 31, 2014, Green Company and Blue Company have identical amounts of common stock and retained earnings as follows: Common Stock, $10 par, 50,000 shares authorized, 9,000 issued, 9,000...
-
The copper coil placed inside a stove with the purpose of heating water that flows through the coil. The coil is made from copper tube with an OD of 1 2 . 7 0 mm and ID of 1 1 . 0 8 mm . Water enters...
-
Confidence Levels Given specific sample data, such as the data given in Exercise 1, which confidence interval is wider: the 95% confidence interval or the 80% confidence interval? Why is it wider?
-
Yellow M&Ms Express the confidence interval (0.0847, 0.153) in the form of P - E < p < p + E. 12. Blue M&Ms Express the confidence interval 0.255 (+-) 0.046 in the form of P - E < p < p + E.
-
An ideal, noble gas with a mass of 97.2 g at 25 C and a pressure of 608 torr has a volume of 22.7 L. 1. What is the pressure (in atm)? SHOW ALL WORK. 2. What is R (number and units)? 3. What is the...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
Describe the usual nature of the following features as they apply to a share of preferred stock: (a) Cumulative, and (b) Convertible.
-
A statistical study shows that the fraction of television sets of a certain brand that are still in service after x years is given by f (x) = e-0.15x. (a) What fraction of the sets are still in...
-
On the basis of the hybrid orbitals involved in the bonds, arrange the bonds in each of the following sets in order of increasing length. C--C single bond of propan; C--C single bond of propyne; C--C...
-
Rank the anions within each series in order of increasing basicity, lowest first. Explain. CH(CH2) CH CH
-
Using simple observations or chemical tests with readily observable results, show how you would distinguish between the compounds in each of the following pairs. (a) l-hexyne and 2-hexyne (b) propyne...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


