Given that the stretching vibration of a typical CH bond has a frequency of about 9 *
Question:
Given that the stretching vibration of a typical C—H bond has a frequency of about 9 * 1013 s–1, which peak(s) in the IR spectrum of nonane (Fig. 12.4) would you assign to the C—H stretching vibrations?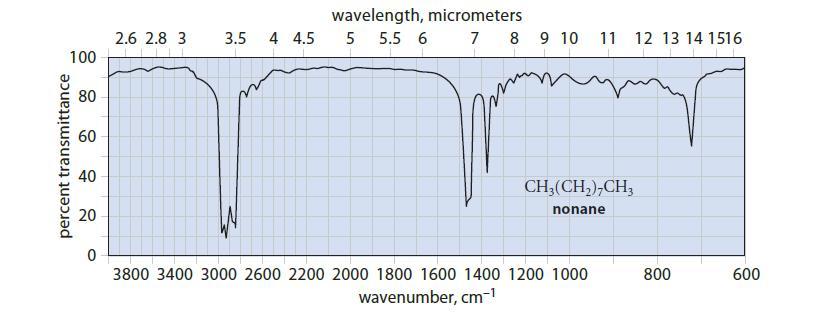
Transcribed Image Text:
percent transmittance 100 80 60 40 20 0 2.6 2.8 3 3.5 4 4.5 wavelength, micrometers 5 5.5 6 7 8 9 10 11 CH₂(CH₂)7CH3 nonane 3800 3400 3000 2600 2200 2000 1800 1600 1400 1200 1000 wavenumber, cm-¹ 12 13 14 1516 800 600
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
From Eq 128a we kn...View the full answer

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which would be expected to be more intense, the stretching vibration of a C==O bond or the stretching vibration of a C==C bond?
-
Given that the force constants are similar for C--H and C--C bonds, explain why the stretching vibration of a bond occurs at a greater wavenumber.
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
You mentioned that Amazon has sustainability topics within us supply chain. How we deal with the criticism that Amazon itself tribute to overconsumption that's antithetical to sustainability
-
Mel Storrer believes there are no significant differences in the flow of costs between job order cost accounting and process cost accounting. Is Storrer correct? Explain.
-
Coakley Beet Processors, Inc., processes sugar beets in batches. A batch of sugar beets costs $47 to buy from farmers and $21 to crush in the company's plant. Two intermediate products, beet fiber...
-
What approaches to valuation should the appraiser consider at the shareholder level?
-
Effective December 31, 2010, Zintel Corporation proposes to issue additional shares of its common stock in exchange for all the assets and liabilities of Smith Corporation and Platz Corporation,...
-
1. In November of 2022 , in Edmonton, Alberta, Canada , the Canadian federal and provincial governments announced approximately $475 million (CAD) in project funding for Air Products'...
-
One of the spectra in Fig. 12.11 is that of trans-2-heptene and the other is that of 2-methyl-1-hexene. Which is which? Explain. 100 percent transmittance (a) 80 60 40 0 2.6 2.8 3 3.5 4 4.5...
-
The base peak in the mass spectrum of 2,2,5,5-tetramethylhexane (molecular mass = 142) is at m/z = 57, which corresponds to a composition C 4 H 9 . (a) Suggest a structure for the fragment that...
-
The Lila Steinman Company is preparing a cash receipts schedule for the first quarter of 2005. Sales on account for November and December 2004 are expected to be \($200,000\) and \($400,000\),...
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
Find the points of intersection of the graphs of y = x2 - 2x + 4 and y - x = 4.
-
What are the key elements of a system investigation report?
-
Hemoglobin has pI = 6.8. Does hemoglobin have a net negative charge or net positive charge at pH = 5.3? At pH = 7.3?
-
Show how you could prepare the following -amino acids from the appropriate carboxylic acids: (a) Phenylalanine (b) Valine
-
What alkyl halides would you use to prepare the following -amine acids by the amidomalonate method? (a) Leucine (b) Histidine (c) Tryptophan (d) Methionine
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


