Given the boiling point of the first compound in each set, estimate the boiling point of the
Question:
Given the boiling point of the first compound in each set, estimate the boiling point of the second.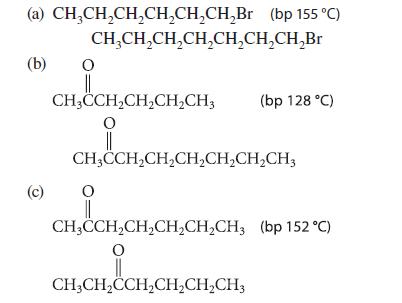
Transcribed Image Text:
(a) CH₂CH₂CH₂CH₂CH₂CH₂Br (bp 155 °C) (b) (c) CH₂CH₂CH₂CH₂CH₂CH₂CH₂Br O CH₂CCH₂CH₂CH₂CH3 2CH₂CH O || CH3CCH₂CH₂CH₂CH₂CH₂CH3 O (bp 128 °C) CH3CCH₂CH₂CH₂CH₂CH3 (bp 152 °C) O CH3CH₂CCH₂CH₂CH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a Use the rule of thumb that an additional carbon ad...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
TIME 1 2 8 AM The following data were collected on the amount of deodorant in cans, in grams, filled in the filling line of a packaging company. Prepare X- and R-charts for the data, and comment on...
-
Estimate the boiling point of a 0.050 m aqueous MgCl2 solution. Assume a value of i based on the formula.
-
Glorious Electrical Appliances (GEP) Co. is a company that sells electrical tools. GEP uses perpetual inventory system in recording its inventory. The financial position of GEP as at 31 December 2016...
-
The Federal Reserve may raise its benchmark interest rate later this month. How is this achieved? Why would they do this? Explain the consequences fully. (Include graphs with your answer)
-
Multiple Choice 1. Colorado passes a hotel tax of 8 percent for Colorado residents and 15 percent for out-of-state visitors. The new law A. Is valid, based on the Supremacy Clause B. Is void, based...
-
Both covalent-network solids and ionic solids can have melting points well in excess of room temperature, and both can be poor conductors of electricity in their pure form. However, in other ways...
-
7. What challenges did the financial crisis of 2008 and its aftermath pose for estimating a firms cost of capital? How should one handle these challenges?
-
Myrtle Company has sales of $140,000, cost of goods sold of $70,000, operating expenses of $20,000, average invested assets of $400,000, and a hurdle rate of 6 percent. Calculate Myrtles return on...
-
Direct Materials and Direct Labor Variances Zoller Company produces a dark chocolate candy bar. Recently, the company adopted the following standards for one bar of the candy: Direct materials (6.20...
-
Draw the structures and give the names of all isomers of octane with (a) Five carbons (b) Six carbons in their principal chains.
-
Give a general balanced reaction for (a) The complete combustion of an alkane (formula C n H 2n+2 ). (b) The complete combustion of a cycloalkane containing one ring (formula C n H 2n ).
-
You work for the strategy group of Adapt Inc., a firm that designs and manufactures memory cards for digital cameras. Your task is to gather intelligence about Adapts key competitor, DigiMem, a...
-
Explain the principles of database normalization and denormalization, delineating their respective roles in optimizing data storage efficiency, query performance, and data integrity in relational...
-
Asymptotic Computational Complexity O(): Calculate the time complexity of each function below and explain your reasoning. Write your answers on paper and submit a scanned copy. (5 pts each) def...
-
Happy Valley Software has developed a new meteorology software package that will likely revolutionize the weather forecasting industry. They are looking to market the software to the following three...
-
Please read the essay Nasty Women Have Much Work To Do from Alexandra Petri on pages 45-47. In your discussion post, please share your thoughts on what specific strategies she uses to create tone and...
-
We live in an increasingly hyper-competitive global marketplace, where firms are fighting to stay lean and flexible in an effort to satisfy increasingly diverse and specialized consumer demand. In...
-
Which characteristics of life can you identify in yourself ?
-
Trade credit from suppliers is a very costly source of funds when discounts are lost. Explain why many firms rely on this source of funds to finance their temporary working capital.
-
Predict the products of the reaction of (i) phenyl acetaldehyde and (ii) acetophenone with the following reagents: (a) NaBH4 then H3O+ (b) Tollens reagent (c) NH2OH, HC1 catalyst (d) CH3MgBr, then...
-
How would you prepare the following substances from 2-cyclohexcnone? More than one step may be required. .CH (a) (b) (d) (c) CSH5 (Two ways)
-
Show how the Wittig reaction might be used to prepare the following alkenes. Identify the alkyl halide and the carbonyl components that would he used. (b) (a)
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


