How many chemically nonequivalent sets of hydrogens are in each of the following structures? (a) CH3CH (c)
Question:
How many chemically nonequivalent sets of hydrogens are in each of the following structures?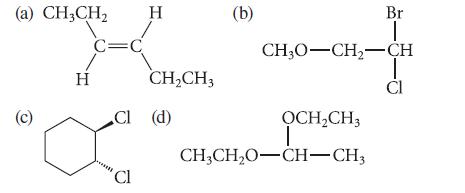
Transcribed Image Text:
(a) CH3CH₂ (c) H C=C CI Cl Cl H CH₂CH3 (d) (b) CH3O-CH₂-CH ỌCH₂CH3 Br T CH3CH₂O-CH-CH3 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a c CH3CH three chemically nonequivalent sets of ...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Show that replacing each of the CH2 protons by some group Q in the (S) enantio-mer of 2-butanol leads to a pair of diastereomers, as it does for the (R) enantiomer. (b) How many chemically...
-
Using the equations your derived in questions 59 and 62 for IS and LM, what will be the equilibrium interest rate (r) and equilibrium output (Y)? A) B) C) D) r = 8, Y = 900B r = 9, Y = 600B r = 10, Y...
-
How many hydrogens are replaced by deuterium when each of the following compounds is treated with NaOD in D2O? a. 3-methylcyclopentanone b. 3-methylhexanal
-
Using the table of implied volatilities below ("volatility surface"), calculate the implied volatility a trader would use for pricing an 8-month option with K/S0 = 1.04. Hint: Interpolate linearly. 1...
-
In the year 2002, Salt Lake City, Utah, hosted the Winter Olympics. To get ready for the Olympics, most of the major roads and highways in and around Salt Lake City were renovated. It took over three...
-
In 2.0 minutes, a ski lift raises four skiers at constant speed to a height of 140 m. The average mass of each skier is 65 kg. What is the average power provided by the tension in the cable pulling...
-
Review the chapter and make a list of all the advantages and disadvantages of matrix project organization LO6 you can find. Then add to the list any additional advantages or disadvantages that may...
-
Johnny Jones?s high school derivatives homework asks for a binomial valuation of a 12- month call option on the common stock of the Overland Railroad. The stock is now selling for $45 per share and...
-
SAGE 5 0 THE FANZI R . COMPANY Fanzi R . opened the FanziCo on January 2 , 2 0 2 2 specialized in sale of 3 sporting goods: Product P 1 , Product P 2 and Product P 3 During the 1 st month of...
-
(a) How many electrons are involved in the oxidation of triphenylphosphine (Ph 3 P;) to triphenylphosphine oxide (Ph 3 P = O)? Show your reasoning. (b) Draw a resonance structure for...
-
How many electrons are involved in the oxidation of 1-propanethiol to each of the following compounds. (See Fig. 10.3 for detailed Lewis structures.) (a) 1-propanesulfonic acid, CH 3 CH 2 CH 2 SO 3 H...
-
Is it possible for a project team to achieve high efficiency without scheduling tasks and activities? Discuss.
-
How do we design an electromagnetic sensor?
-
What is a virtual breadboard?
-
Joe secured a loan of $13,000 four years ago from a bank for use toward his college expenses. The bank charges interest at the rate of 9%/year compounded monthly on his loan. Now that he has...
-
Answer these two questions 1 32 2 Number of Units Sold 3 4 ! Direct Material units per unit of production 5 i 6 Total Direct Materials Used 7! 8 Price Per Unit 9 10 Cost of Direct Materials 11 12 13...
-
Give an algorithm for converting a tree to its mirror. Mirror of a tree is another tree with left and right children of all non-leaf nodes interchanged. The trees below are mirrors to each other....
-
The relationship between the unit price P (in cents) for a certain product and the demand D (in thousands of units) appears to satisfy P = 29 - 3D + D2 On the other hand, the demand has risen over...
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
Identify the following intermediate in the citric acid cycle, and tell whether it has R or Sstereochemistry:
-
The following compound is an intermediate in the biosynthesis of one of the twenty common a-amino acids. Which one is it likely to be, and what kind of chemical change must take place to complete...
-
The following compound is an intermediate in the pentose phosphate pathway, an alternative route for glucose metabolism. Identify the sugar it is derivedfrom.
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


