Identify each of the compounds AD in the reaction scheme shown in Fig. P27.76. Explain your answers.
Question:
Identify each of the compounds A–D in the reaction scheme shown in Fig. P27.76. Explain your answers.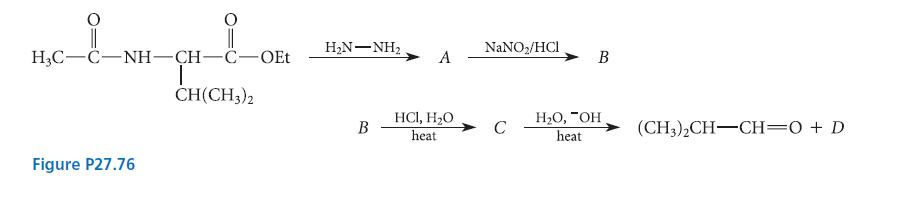
Transcribed Image Text:
i || || H3C-C-NH-CH-C-OEt I CH(CH3)2 Figure P27.76 HN–NH, B A HC1, H₂O heat NaNO₂/HCI C B H₂O, "OH heat (CH3)2CH-CH=0 + D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The chemistry is very similar to that of Problem 2775i text ...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify each of the compounds A-D in the reaction scheme shown in Fig. P26.67, p. 1331. Explain your answers. H,N NH NaNO /HCI CH(CH3)2 HCL H20 C -heat (CHs)-CH-CH-=O + D
-
Identify each of the following compounds as aromatic, nonaromatic, or antiaromatic. Explain your choice in each case. a. b. c. d. e. f. g. h. :N-H
-
Identify each of the following compounds from the NMR data and molecular formula. The number of hydrogens responsible for each signal is shown in parentheses. a. C4H8Br2 1.97 ppm (6) singlet 3.89 ppm...
-
A mail-order firm processes 5,300 checks per month. Of these, 60 percent are for $55 and 40 percent are for $80. The $55 checks are delayed two days on average; the $80 checks are delayed three days...
-
For each of the following items, enter the correct letter to the left to show the type of expenditure. Use thefollowing: Type of Expenditure Capital expenditure Revenue expenditure Neither...
-
Give at least two organizational examples of unethical behavior and the justification that was used in each instance.
-
At the end of each year a self-employed person deposits $1,500 in a retirement account that earns 7 percent annually. a) How much will be in the account when the individual retires at the age of 65...
-
Amalgamated Corporation, organized under the laws of State S, sends several traveling salespersons into State M to solicit orders, which are accepted only at the home office of Amalgamated...
-
Please give an explanation. 2 answers are correct 2 are wrong O Assuming the given periodic profitability summary Mobiles TV 000s E000s Sales 1000 900 Variable Costs (528) (598) Fixed Costs (318)...
-
Draw a curved-arrow mechanism for each of the reactions given in Fig. P27.77. (a) (b) (c) (d) CH=O CH=0 o-phthalaldehyde CH 0 I CHCl + NH3 + Na+ SH + acetone H + HN-CH-CO2 + HSCHCHOH CH3 Figure...
-
(a) For many years it was difficult to determine the X-ray structures of proteins that are imbedded in membranes because, when they are extracted into an aqueous buffer, they denature. Explain why...
-
Find all real solutions. Check your results. 35 4 X +15
-
Because her insurance agency is in a lakeside community, Adriana always asks her homeowners clients what boating activities they engage in, and she is sure to add the Watercraft endorsement...
-
1. Among all assumptions in CVP analysis, which one do you think is the most critical? Explain. 2. How will you change the cost-volume-profit analysis if the assumption (you identify in the previous...
-
Construct a confidence interval for p-P2 at the given level of confidence. x =26, n =229, x2 = 31, n = 302, 95% confidence The researchers are % confident the difference between the two population...
-
AP 9-2 (Moving Expenses) In May of the current year, following a dispute with her immediate superior, Ms. Elaine Fox resigned from her job in Halifax and began to look for other employment. She was...
-
Minimize the number of states in the following DFA: A b b a a a b b b E B a a
-
The management of East Coast Railroad Company introduced in Exercise improved the profitability of the Atlanta/Baltimore route in May by reducing the price of a railcar from $600 to $500. This price...
-
Let X be a random variable taking on values a1, a2, . . . , pr with probabilities p1, p2, . . . , pr and with E(X) = μ. Define the spread of X as follows: This, like the standard deviation, is a...
-
Using Table 3.1, as well as the data given below, estimate the equilibrium constants for the following reactions at 25C. pK, 10.5
-
What is the standard free-energy change at 25C for reaction (b) in Problem 3.40?
-
Phenylacetic acid has a pKa of 4.31; acetic acid has a pKa of 4.76. phenylacetic acid acetic acid
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


