Identify the compound C 4 H 9 NO with the proton NMR spectrum given in Fig. 21.3.
Question:
Identify the compound C4H9NO with the proton NMR spectrum given in Fig. 21.3. This compound has IR absorptions at 3300 and 1650 cm–1.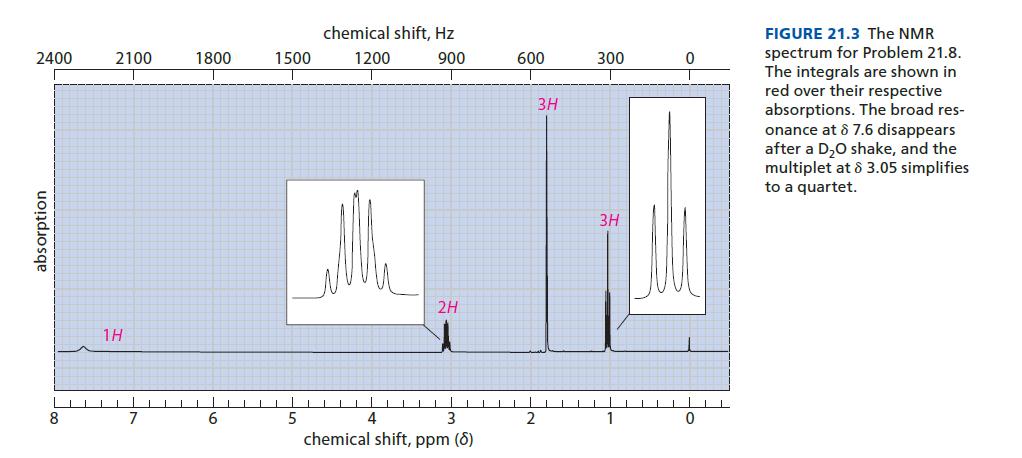
Transcribed Image Text:
2400 absorption 8 2100 18 7 1800 6 1500 5 chemical shift, Hz 1200 900 T 2H 4 3 chemical shift, ppm (8) 600 2 3H 300 3H 1 0 0 FIGURE 21.3 The NMR spectrum for Problem 21.8. The integrals are shown in red over their respective absorptions. The broad res- onance at 8 7.6 disappears after a D₂O shake, and the multiplet at 8 3.05 simplifies to a quartet.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
That the compound is an amide is confirmed by the NH ab...View the full answer

Answered By

Sumit kumar
Education details:
QUATERNARY Pursuing M.Tech.(2017-2019) in Electronics and Communication Engg. (VLSI DESIGN) from
GNIOT Greater Noida
TERTIARY B.Tech. (2012-2016) in Electronics and Communication Engg. from GLBITM Greater Noida
SECONDARY Senior Secondary School Examination (Class XII) in 2012 from R.S.S.Inter College, Noida
ELEMENTARY Secondary School Examination (Class X) in 2010 from New R.J.C. Public School ,Noida
CERTIFICATION
Summer Training in ‘WIRELESS EMBEDDED SYSTEM’ from ‘XIONEE’ for the six weeks.
EMBEDDED SYSTEM Certificate issued by CETPA INFOTECH for one day workshop.
Certificate of Faculty development program on OPTICAL COMMUNICATION and NETWORKS for one week.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the compound with the mass spectrum and proton NMR spectrum shown in Fig. P19.67. This compound has IR absorptions at 1678 cm 1 and 1600 cm 1 . 100 183 185 155 60 40 1157 43 198 200 La fl 0...
-
(a) Propose a structure for an amine A (C 4 H 9 N), which liberates a gas when treated with NaNO 2 and HCl. The 13 C NMR spectrum of A is as follows, with attached protons in parentheses: 14(2), ...
-
Propose structures for compounds that meet the following descriptions: (a) C5H8, with IR absorptions at 3300 and 2150 cm1 (b) C4H80, with a strong IR absorption at 3400 cm1 (c) C4H80, with a strong...
-
Write C++ statements to do the following. a. Declare int variables num1 and num2. b. Prompt the user to input two integers. c. Input the first number in num1 and the second number in num2. d. Output...
-
Review the data given in Exercise 5-21. Requirement 1. Prepare Budgets single-step income statement.
-
Gateway Construction Company is a family-operated business that was founded in 1950 by Samuel Gateway. In the beginning, the company consisted of Gateway and three employees laying gas, water, and...
-
Suppose that Merton plc (see Example 9.1 ) replaces the 30,000 paid out as dividends by an issue of shares to new shareholders. Show the statement of financial position after the new issue and...
-
High correlation between two variables means that one is the cause and the other is the effect. Do you agree? Explain.
-
Ethics Case-Granting Contracts Leslie Haley holds a senior human resources position in a large business. Leslie's responsibilities include health insurance contracts with insurance providers. For...
-
(a) Assuming that the difference in the relative boiling points of methyl acetate and 2-butanone (see display above) is caused by the difference in their dipole moments, predict which compound has...
-
Draw the structure of an amide that must exist in an E conformation about the carbonylnitrogen bond.
-
1. If the elevation of Lake Superior is 600 feet above sea level and the elevation of the Caspian Sea is 92 feet below sea level, find the difference of the elevations. 2. Josh Weidner has $142 in...
-
On October 1, Deloitte \& Coopers Price started a consulting firm. The asset, liability, and stockholders' equity account balances after each of the firm's first six transactions are shown below....
-
On June 1, a group of bush pilots in British Columbia, Canada, formed the Adventure Airlines, Inc., by selling \(\$ 51,000\) of common stock for cash. The group then leased several aircraft and...
-
During the first year of operation, 2011, Martin's Appliance recognized \$292,000 of service revenue on account. At the end of 2011 , the accounts receivable balance was \(\$ 57,400\). Even though...
-
During May, Willett Corp. purchased direct materials for 4,250 units at a total cost of \($61,625\). Willetts standard direct materials cost is \($14\) per unit. Prepare the journal entry to record...
-
Determine a positive real root of this equation using appropriate software: \[ 3.5 x^{3}-10 x^{0.5}-3 x=-4 \]
-
Solve the following initial value problems: (a) (b) (c) (d) (e) (f) (g) du (0 2 2 0 u, U 0 du di2 I 1-2 )u, u(0)=( 4 2 1 4 2)u, u(0) 0 1 (0 du du dt 0 3-4 0 2-6 dt 0-12 10 0 0 2.. u(2) du dt 0 2 00/...
-
[a] Two foam blocks, each with a charge of 19 micro coulombs (1 C = 10-6 C), are both held in place 19 cm apart in the east-west direction. A foam ball with a charge 49 C is placed 55 cm north of the...
-
Which diene and dienophile would you employ to synthesize the following compounds? (a) (b) CO2Me CO2Me
-
Diels-Alder reactions also take place with triple-bonded (acetylenic) dienophiles. Which diene and which dienophile would you use to prepare the following? CO2Me CO2Me
-
1,3-Butadiene and the dienophile shown below were used by A. Eschenmoser in his synthesis of vitamin B12 with R. B. Woodward. Draw the structure of the enantiomeric Diels-Alder adducts that would...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


