Identify the compound with the mass spectrum and proton NMR spectrum shown in Fig. P19.67. This compound
Question:
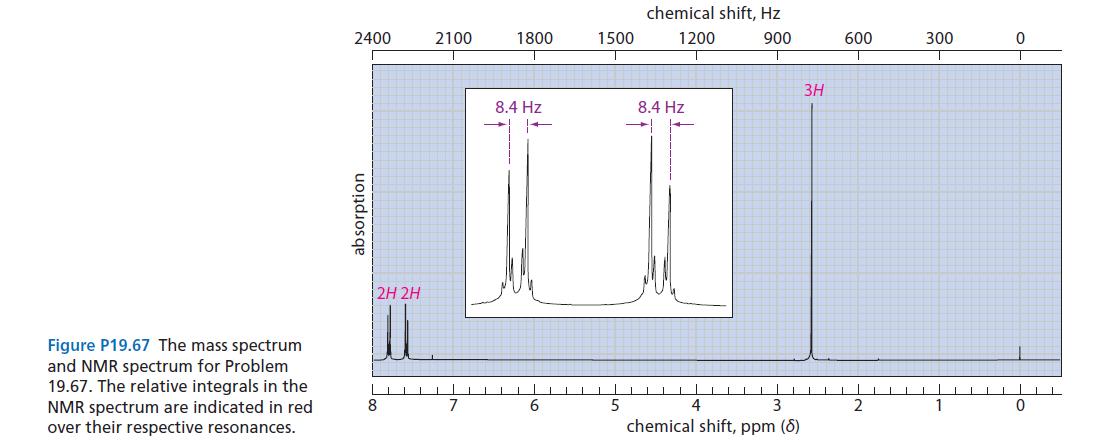
Identify the compound with the mass spectrum and proton NMR spectrum shown in Fig. P19.67. This compound has IR absorptions at 1678 cm–1 and 1600 cm–1.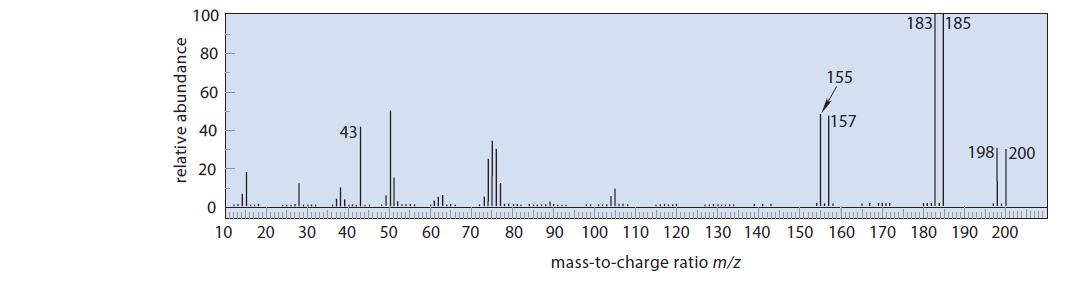
Transcribed Image Text:
100 183 185 155 60 40 1157 43 198 200 La fl 0 10 20 30 40 50 60 70 150 160 170 180 190 200 80 90 100 110 120 130 140 mass-to-charge ratio m/z
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The double absorptions of equal intensity in the mass spectrum indicate the presence ...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the compound C 4 H 9 NO with the proton NMR spectrum given in Fig. 21.3. This compound has IR absorptions at 3300 and 1650 cm 1 . 2400 absorption 8 2100 18 7 1800 6 1500 5 chemical shift, Hz...
-
Journalize the following transactions (the company is using a perpetual system). 1. On June 1, ABC Co.Purchased 50 T-shirts at $5 each from Neon Textile Co, credit terms 2/10, n/30. (On Account) DATE...
-
Identify the compound with molecular formula C8H10O that gives the IR and 1H NMR spectra shown in Figure 14.23. 23 16 27 2 29 35 13 14 15 16 800 200 2400 200 0 6 (ppm) frequency
-
Plastic Co Pte Ltd (Plastico) is a Fiji company. The company has issued and paid up capital of $250,000 held in equal parts by two brothers. The companys business involves making plastic products for...
-
Selected accounts of Guitars by Peter, Inc., for the year ended December 31, 2012, follow: Requirement 1. Prepare the companys statement of retained earnings for theyear. Retained carnings Income...
-
Solve each of the following parts independently. 1. Lowney Limited has hired a public accounting firm to identify opportunities to reduce their corporate income tax expense. The firm's tax advisory...
-
Length of pregnancies. The length of human pregnancies from conception to birth varies according to a distribution that is approximately Normal with mean 266 days and standard deviation 16 days. Use...
-
Brunswick Parts is a small manufacturing firm located in eastern Canada. The company, founded in 1947, produces metal parts for many of the larger manufacturing firms located in both Canada and the...
-
part 3 Required information [The following information applies to the questions displayed below) University Car Wash built a deluxe car wash across the street from campus. The new machines cost...
-
(a) You are the chief organic chemist for Bugs and Slugs, Inc., a firm that specializes in environmentally friendly pest control. You have been asked to design a synthesis of 4-methyl-3-heptanol, the...
-
Identify the following compounds. (a) C0H0O NMR: 8 2.82 (6H, s), 8 8.13 (4H, s) IR: 1681 cm-, no 0-H stretch (b) C-H0ONMR: 8 9.8 (1H, s), 8 1.1 (9H, s) NMR in Fig. P19.66 (p. 1002) IR: 1701 cm-, 970...
-
Apamine is a small protein toxin present in the venom of the honeybee. It has the sequence CNCKAPETALCARRCQQH (a) If apamine does not react with iodoacetate (see Tools of Biochemistry 5B), then how...
-
2. Getting ready for Logarithms and Calculus! a. Fill in the chart and graph the function (I advise practicing on your scientific calculator and desmos. X f(x) = Inx 0 0.5 1 e 10...
-
JoJo Co. had the following balances and information for October. Beg. finished goods inventory = $30 Beg. work in process inventory = $5 Beg. raw materials inventory = $15 End. finished goods...
-
Subway sales have been declining since 2014. In the US, Subway has closed a number of stores due to over-expansion, outdated operations, and uninspiring menus. In Canada, Subway took a different...
-
Harvey Auto Parts purchased a new crane on September 1 for $35,000, paying $10,000 cash and signing a 7%, 12-month note for the remaining balance, interest to be paid at maturity. The crane is...
-
e4(k+1) Find the sum of the series. k = 1 8
-
Let AT = -A be a real, skew-symmetric n n matrix. (a) Prove that the only possible real eigenvalue of A is = 0. (b) More generally, prove that all eigenvalues of A are purely imaginary, i.e., Re ...
-
The Place-Plus real estate development firm in Problem 24 is dissatisfied with the economists estimate of the probabilities of future interest rate movement, so it is considering having a financial...
-
What product would be obtained if A were treated with lithium aluminum hydride without first converting it to a cyclic acetal?
-
(a) Show how you might use a cyclic acetal in carrying out the following transformation: (b) Why would a direct addition of methylmagnesium bromide to A fail to give B? OH
-
Dihydropyran reacts readily with an alcohol in the presence of a trace of anhydrous HCl or H2SO4 to form a tetrahydropyranyl (THP) ether: (a) Write a plausible mechanism for this reaction. (b)...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


