Identify the compound with a molecular mass of 82 that has the IR spectrum shown in Fig.
Question:
Identify the compound with a molecular mass of 82 that has the IR spectrum shown in Fig. 14.7 and the following NMR spectrum: δ 1.90 (1H, s); δ 1.21 (9H, s).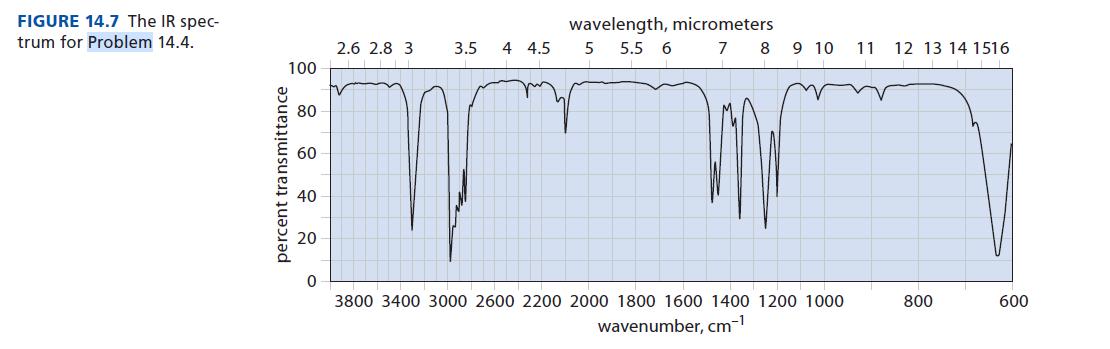
Transcribed Image Text:
FIGURE 14.7 The IR spec- trum for Problem 14.4. 100 percent transmittance 80 60 40 20 2.6 2.8 3. 3.5 4 4.5 wavelength, micrometers 5 5.5 6 7 8 9 10 3800 3400 3000 2600 2200 2000 1800 1600 1400 1200 1000 wavenumber, cm-¹ 11 12 13 14 1516 800 600
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
A nineproton singlet cries out tertbutyl group The other reson...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the compound with molecular formula C8H10O that gives the IR and 1H NMR spectra shown in Figure 14.23. 23 16 27 2 29 35 13 14 15 16 800 200 2400 200 0 6 (ppm) frequency
-
Wells Fargo blaming its 5,000 employees who were fired for falsifying accounts as purely their own doing and ignoring the role their own company rewards program played, is an example of ____. Group...
-
Identify the compound with molecular formula C3H7NO responsible for the 1H NMR spectrum in Figure 14.29. 0 5
-
Make a Marketing Plan for business during the Covid 19 Pandemic as it is today. Position yourself as an entrepreneur looking to open a new business or expand your existing business. Use assumptions...
-
Total fixed costs are $25,000 for Haag Inc. It has a contribution margin per unit of $15, and a contribution margin ratio of 25%. Compute the break-even sales in dollars.
-
In an experiment to study the way in which different anesthetics affect plasma epinephrine concentration, ten dogs were selected and concentration was measured while they were under the influence of...
-
In valuing illiquid interests in private businesses, why do appraisers normally begin with appraisals at the marketability minority level of value?
-
Using the Yahoo! Finance Web site (finance.yahoo.com) get the current price and five year dividend history for Intel. To gather this data, enter the ticker symbol (INTC) in the search box at the top...
-
The bottom-up budget development approach the budget: None of these. C. process begins with the issuance of general guidelines by top management. D. Both B and C. A. is imposed on lower-level...
-
From which alkyne could each of the following compounds be prepared by acid- catalyzed hydration? (a) (c) CH3CCHCHCH3 (b) O (CH3)3C-C-CH3
-
(a) Match each of the following 13 C NMR spectra to either 2-hexyne or 3-hexyne. Explain. Spectrum A: 3.3, 13.6, 21.1, 22.9, 75.4, 79.1 Spectrum B: 12.7, 14.6, 81.0 (b) Assign each of the...
-
A medical statistician wants to estimate the average weight loss of people who are on a new diet plan. In a preliminary study, he guesses that the standard deviation of the population of weight...
-
At March 31, account balances after adjustments for Vizzini Cinema are as follows: Account Balances Accounts Cash Supplies Equipment (After Adjustment) $11,000 4,000 50,000 Accumulated...
-
2. "A student holds a thin aluminum pie pan horizontally 2 m above the ground and releases it. Using a motion detector, she obtains the graph shown in Figure P3.12. Based on her measurements, (a)...
-
Mark has two sticks, 25 inches, and 20 inches. If he places them end-to-end perpendicularly, what two acute angles would be formed when he added the hypotenuse?
-
A wedding website states that the average cost of a wedding is $29,205. One concerned bride hopes that the average is less than reported. To see if her hope is correct, she surveys 36 recently...
-
2. (10 pts each) Use partial fractions decomposition and the tables to find the inverse z- transform of each of the following: a. X(z)= 6z-z z3-4z2-z+4 4z2 b. G(z)=- (z-1) (z-0.5) 3z +1 c. X(z) =...
-
Let f(x) = x - 1 / x and g(x) = x2 + 1. Find each value. (a) (f + g) (2) (b) f g) (2) (c) (f g) (2) (d) (g f) (2) (e) f3 (- 1) (f) f2(2) + g2 (2)
-
Calculate I, , and a for a 0.0175 m solution of Na 3 PO 4 at 298 K. Assume complete dissociation. How confident are you that your calculated results will agree with experimental results?
-
Galactose, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized from UDP-glucose by galactose 4-epimerase, where UDP = uridylyl diphosphate (a ribonucleotide diphosphate;...
-
Mannose, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized as its 6-phosphate derivative from fructose 6-phosphate. No enzyme cofactor is required. Propose a mechanism....
-
Glucosamine, one of the eight essential monosaccharide?s (Section 25.7), is biosynthesized as its 6-phosphate derivative from fructose 6-phosphate by reaction with ammonia. Propose a mechanism....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


