In 1960, a group of chemists led by Prof. John D. Roberts at Caltech reported that when
Question:
In 1960, a group of chemists led by Prof. John D. Roberts at Caltech reported that when chlorobenzene containing a 14C isotopic label at carbon-1 is treated with the very strong base potassium amide, a substitution product, aniline, is formed in which the isotopic label is equally distributed between carbon-1 and carbon-2. This result was interpreted as evidence for a very interesting unstable intermediate called benzyne. Propose a mechanism for this substitution that accounts for the isotopic labeling result.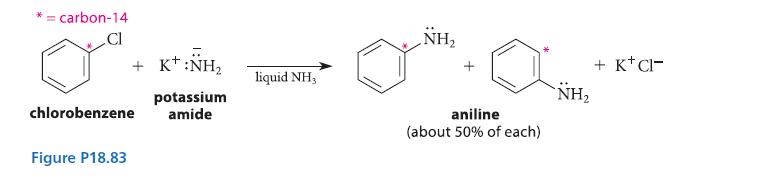
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





