In each case, draw the structure of the cyclic anhydride that forms when the dicarboxylic acid is
Question:
In each case, draw the structure of the cyclic anhydride that forms when the dicarboxylic acid is heated.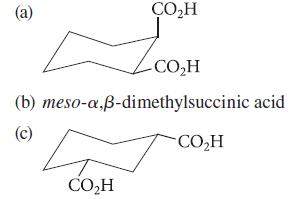
Transcribed Image Text:
(a) (b) (c) CO₂H 4 CO₂H CO₂H meso-a,ß-dimethylsuccinic acid CO₂H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a b H3C H3C H ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the structure of the cyclic anhydride that forms when the following acids is heated. meso-,-dimethylsuccinic acid
-
Draw the structure of the cyclic compound that is produced when acetone is treated with 1,3 propanedithiol in the presence of an acid catalyst. SH 1,3-propanedithiol HS Acetone
-
The following triene reacts with excess maleic anhydride to produce a compound with molecular formula C 14 H 12 O 6 . Draw the structure of this product (ignoring stereochemistry). Maleic anhydride...
-
Q3: Prove: For any sets A and B, Ax B = B A ?
-
Carolina Communications, Corp., reported the following figures in its financial statements: Requirement 1. Prepare the businesss multi-step income statement for the year ended July 31,2012. S 3,800...
-
Jelkin Corporation is a processor of apples. The manufacturing facility has two divisions: the puree division and the finishing division. As apples arrive at the facility, the puree division cleans...
-
Can you think what these reasons may be?
-
Xemex has collected the following inventory data for the six items that it stocks: Lynn Robinson, Xemexs inventory manager, does not feel that all of the items can be controlled. What ordered...
-
Allure Company manufactures and distributes two products, M and XY Overhead costs are currently allocated using the number of units produced as the allocation base. The controller has recommended...
-
(a) Organolithium reagents such as methyllithium (CH 3 Li) react with carboxylic acids to give ketones. Two equivalents of the lithium reagent are required, and the ketone does not react further....
-
You are a chemist for Chlorganics, Inc., a company specializing in chlorinated organic compounds. A process engineer, Turner Switchback, has accidentally mixed the contents of four vats containing,...
-
A corporation reacquires 25,000 shares of its own $10 par common stock for $1,000,000, recording it at cost. a. What effect does this transaction have on revenue or expense of the period? b. What...
-
The following information is available for two different types of businesses for the 2011 accounting period. Dixon Consulting is a service business that provides consulting services to small...
-
Marino Basket Company had a \(\$ 6,200\) beginning balance in its Merchandise Inventory account. The following information regarding Marino's purchases and sales of inventory during its 2011...
-
On March 6, 2011, Bob's Imports purchased merchandise from Watches Inc. with a list price of \(\$ 31,000\), terms \(2 / 10, n / 45\). On March 10, Bob's returned merchandise to Watches Inc. for...
-
The following events apply to Tops Gift Shop for 2012, its first year of operation: 1. Acquired \(\$ 45,000\) cash from the issue of common stock. 2. Issued common stock to Kayla Taylor, one of the...
-
Indicate whether each of the following costs is a product cost or a period (selling and administrative) cost. a. Transportation-in. b. Insurance on the office building. c. Office supplies. d. Costs...
-
Show that a real unitary matrix is an orthogonal matrix.
-
Suppose that a business sells 6-month subscriptions to its monthly magazine. On January 1, the company receives a total of $600 for 10 subscriptions. To record this transaction, the company debits...
-
Draw the structure of the product from the following reaction (formed during a synthesis of one of the endiandric acids by K. C. Nicolaou): MeO2C osi(t-Bu)Phe toluene, 110C
-
Draw all of the contributing resonance structures and the resonance hybrid for the carbocation that would result from ionization of bromine from 5-bromo-1,3-pentadiene. Open the computer molecular...
-
(a) Which other compounds in Section 13.5 are conjugated dienes? (b) Which other compounds are isolated dienes? (c) Which compound is an isolated enyne?
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


