The following triene reacts with excess maleic anhydride to produce a compound with molecular formula C 14
Question:
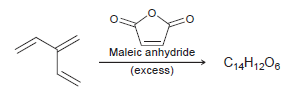
Transcribed Image Text:
Maleic anhydride (excess) C14H1208
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider a process that attempts to prepare tyrosine using a HellVolhardZelinski reaction: (a) Identify the necessary starting carboxylic acid. (b) When treated with Br 2 , the starting carboxylic...
-
Hydrocarbon A, C 10 H 14 , has a UV absorption at ? max = 236 nm and gives hydrocarbon B, C 10 H 18 , on catalytic hydrogenation. Ozonolysis of A followed by zinc/acetic acid treatment yields the...
-
A mixture of maleic anhydride and benzoic acid containing 10 mol% acid is a product of the manufacture of phthalic anhydride. The mixture is to be distilled continuously in a column with a total...
-
On September 1, 2025, Swifty Corporation acquired Windsor Enterprises for a cash payment of $800,000. At the time of purchase, Windsor's balance sheet showed assets of $570,000, liabilities of...
-
The state of plane stress in a body is described by the following stresses: 1 = 8500 kN/m2 compression, 3 = 1500 kN/m2 tension. Determine by means of the Mohr circle the normal stress and shear...
-
[-/1 Points] DETAILS MY NOTES ASK YOUR TEACHER The greatest ocean depths on the Earth are found in the Marianas Trench near the Philippines, where the depth of the bottom of the trench is about 11.0...
-
3. What are the duties of the U.S. trustee under BAPCPA? Do U.S. trustees supervise the administration of all bankruptcy cases?
-
Sim Corporation, a 90 percent-owned subsidiary of Pal Corporation, was acquired on January 1, 2011, at a price of $45,000 in excess of underlying book value. The excess was due to goodwill. Separate...
-
Economic Value Added Washington Company has two divisions: the Adams Division and the Jefferson Division. The following information pertains to last year's results: Adams Division Jefferson Division...
-
You have an opportunity to purchase a 5-story multifamily building which is presently vacant. The asking price is $500,000. After discussing the project with your architect and general contractor,...
-
Government inspectors who check on the quality of services provided by retailers as well as government requirements for licensing in various professions are both attempts to resolve: a. The moral...
-
True or False: A market may collapse and have relatively few transactions between buyers and sellers if buyers have more information than sellers.
-
Solve. Write answers in standard form. x +5=0
-
Propose how these mechanisms can be used to build a strategic business partnership, close the gap between management / leadership and employees while building a cohesive culture that adds value,...
-
1.What risks does the company face? 2. What is role for ERM at Swissgrid or most any company? 3. What risk management processes has Meyer installed at Swissgrid? Assess their strengths and...
-
Elizabeth's Country Wares How many workers does Elizabeth have and what does each of them do? What type of work does Elizabeth do for the CP product line? How long does it take to do the underglazing...
-
Do you support the policy of not allowing some Chinese nationals to attend graduate school in the United States because of national security concerns?
-
Using your product or service name or category, do a search using the following phrase: Find a (insert the name of your product or service here...) near me. For instance, using my Mobile Notary...
-
In Problems 3760, graph each function using the techniques of shifting, compressing, stretching, and/or reflecting. Start with the graph of the basic function (for example, y = x 2 ) and show all the...
-
Phosgene, COCl2, is a toxic gas used in the manufacture of urethane plastics. The gas dissociates at high temperature. At 400oC, the equilibrium constant Kc is 8.05 104. Find the percentage of...
-
Why do you suppose ketone halogenations in acidic media referred to as being acid-catalyzed, whereas halogenations in basic media are base-promoted? In other words, why is a full equivalent of base...
-
How could you use a malonic ester synthesis to prepare the following compounds? Show allsteps. (b) CH3CH2CH2CHC CH (c) CHCH2C CHCHCH-CH,C C
-
Monoalkylated and dialkylated acetic acids can be prepared by the malonic ester synthesis, but trialkylated acetic acids (R3CCO2H) cant be prepared. Explain
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


