In each of the following pairs, one compound has a melting point that is much higher than
Question:
In each of the following pairs, one compound has a melting point that is much higher than the other, but the two have very similar boiling points. Choose the compound with the greater melting point, and explain your reasoning.
(a) (reported melting points 62–65°C, 17°C)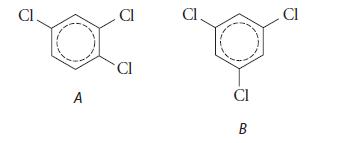
(b) (reported melting points 61–62°C, 21°C)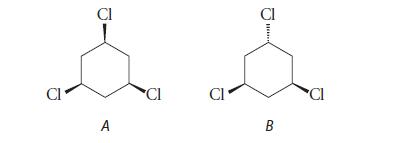
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





