In some tRNAs the anticodon contains an inosine. The heterocyclic base in inosine is hypoxanthine. Inosine can
Question:
In some tRNAs the anticodon contains an inosine. The heterocyclic base in inosine is hypoxanthine.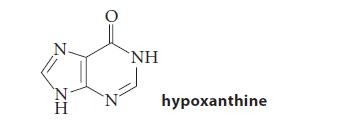
Inosine can form hydrogen-bonded base pairs with A, U, or C. This means that the inosine in tRNA can pair any of these bases in mRNA. Show the hydrogen-bonded base pair of hypoxanthine
(a) With adenine;
(b) With uracil.
Transcribed Image Text:
HZ N ΝΗ hypoxanthine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a hypoxanthine H OHN CH OHIN N B...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Proteins are synthesized with a particular amino acid sequence through the translation of information encoded in messenger RNA by an RNAprotein complex called a ribosome. Amino acids are specified by...
-
The ribonucleosides that make up ribonucleic acid (RNA) are composed of D-ribose (a sugar) and four heterocyclic bases. The general structure of a ribonucleoside is The four heterocyclic bases are...
-
The compound known as nitrous acid is a reactive chemical that replaces amino groups (-NH2) with keto groups (=O). When nitrous acid reacts with the bases in DNA, it can change cytosine to uracil and...
-
On December 1, 2011, Lavender Manufacturing Company (a corporation) purchased another company's assets, including a patent. The patent was used in Lavender's manufacturing operations; $49,500 was...
-
Coke and Pepsi are well-known international brands. Coca-Cola sells more than $13 billion worth of beverages each year while annual sales of PepsiCo products exceed $22 billion. Compare the two...
-
Genetic linkage studies can usually only roughly locate the chromosomal position of a "disease" gene. How can expression analysis and DNA sequence analysis help locate a disease gene within the...
-
Explain the three steps of the planning phase of the strategic marketing process. LO.1
-
The adjusted trial balance of Honeybell, Inc., follows. Requirement Prepare Honeybell, Inc.'s single-step income statement and statement of retained earnings for the year ended December 31, 2016, and...
-
Question 17 In its first year of operations, Lima Company manufactured 500 widgets, incurring direct materials and labor costs of $100,000. For book purposes, Lima capitalized $50,000 of indirect...
-
AQC-tryptophan is not shown in Fig. 27.5 because the indole ring does not survive the acid hydrolysis. In what general region of the chromatogram would you expect to find AQC-Trp if it were present?...
-
What average yield per amino acid would be required to synthesize a protein containing 100 amino acids in 50% overall yield?
-
Bill has asked you to prepare financial statements for his first month of operation. The following information relates to the month of May, 2014. (Round all calculations to the nearest whole dollar)...
-
The relevance ( Relevance ) and the reliability ( Reliability ) represent two characters Key qualitative statistics of information n accountant. What What do these two mean? terms in an accounting...
-
A virtual memory system has a page size of 1024 bytes, six virtual pages, and five physical page frames. The page table is shown in Table Q2(d) as follows: Virtual Page Number (VPN) 0 Page Frame...
-
31. z = x + 2xy, determine which of (I)-(II) in Figure 12.31 are cross- sections with x fixed and which are cross-sections with y fixed. (1) (11) -2 -2+
-
WHAT DOES SOCIETY EXPECT FROM ORGANIZATIONS AND MANAGERS? Introduction: TOMS Shoes has a unique idea to promote corporate social responsibility. For each pair of shoes it sells, it donates a pair to...
-
1. A car heading east turns right at a corner. The car turns at a constant speed of 20.0 m/s. After 12 s, the car completes the turn, so that it is heading due south at 20.0 m/s. Calculate the car's...
-
DiSalvio Co. uses a job order cost system. The following data summarize the operations related to production for May: a. Materials purchased on account, $634,000. b. Materials requisitioned,...
-
In muscle tissue, the ratio of phosphorylase a to phosphorylase b determines the rate of conversion of glycogen to glucose 1phosphate. Classify how each event affects the rate of glycogen breakdown...
-
Which of the following alcohols can be synthesizecl relatively free of constitutional isomers and diastereomers by oxymercuration-reduction? Explain. H,C CH,CH2CH CH2CH CH H,C H,C
-
For each of the following reactions. provide the following information. (a) Give the structures of all products (including stereoisomers). (b) If more than one product is formed, give the...
-
Draw the structures of the following compounds. (Some parts may have more than one correct answer.) (a) An achiral trimethylcyclohexane with two chair forms that are conformational diastereomers. (b)...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


