AQC-tryptophan is not shown in Fig. 27.5 because the indole ring does not survive the acid hydrolysis.
Question:
AQC-tryptophan is not shown in Fig. 27.5 because the indole ring does not survive the acid hydrolysis. In what general region of the chromatogram would you expect to find AQC-Trp if it were present? Explain.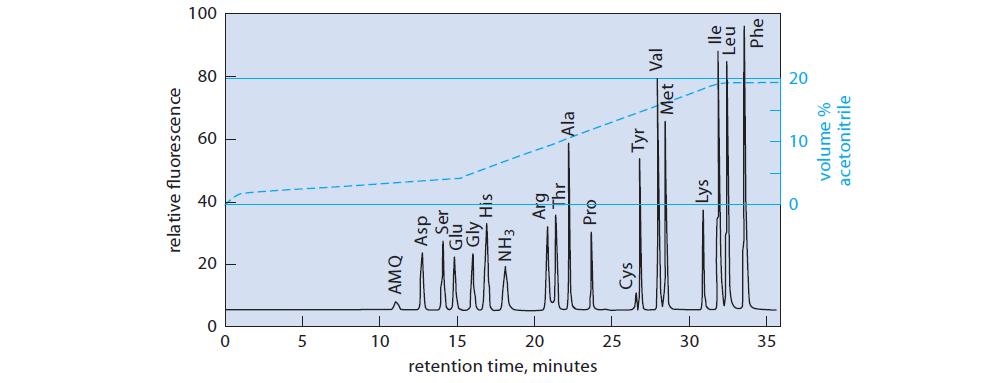
Transcribed Image Text:
0 5 10 15 retention time, minutes 20 25 30 35 20 AMQ relative fluorescence Cys Asp Ser Glu Gly NH3 His Arg Thr Pro Lys 60 Ala Tyr 80 Met volume % acetonitrile Val 8 lle Leu 100 Phe
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The indole sidechain of tryptophan is very h...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Because federal law only requires disclosure of all material information, who bears the responsibility for ensuring the quality and fairness of an offering? The managers and directors of the issuing...
-
AQC-typtoptan is not shown in Fig. 26.3, in what general region of the chromatogram would you expect to find AQC-Trp if it ware preseirr? Explain.
-
What amino acid residues would you expect to find at the cytochrome c-binding sites of Complex III and Complex IV?
-
Dennis Harding is considering acquiring a new automobile that he will use 100% for business. The purchase price of the automobile would be $48,500. If Dennis leased the car for five years, the lease...
-
Winter Corporation has just completed its comparative statements for the year ended December 31, 2012. At this point, certain analytical and interpretive procedures are to be undertaken. The...
-
What is the name given to the process that can repair DNA damage and generate genetic diversity? Briefly describe the similarities and differences of the two processes.
-
Discuss how an organization assesses where it is now and where it seeks to be. LO.1
-
Scores on a marketing exam are known to be normally distributed with mean and standard deviation of 60 and 20, respectively. a. Find the probability that a randomly selected student scores between 50...
-
From the following information, Calculate Labour Rate Variance and Labour Efficiency Variance : Standard rate per hour 4.00 Standard Time per Unit of Output 20 hours Units Produced 500 Actual Hours...
-
(a) Notice in peptide P (see previous discussion) that Asn and Asp are not distinguished by amino acid analysis. Explain. (b) What other pair of amino acids are not differentiated by amino acid...
-
In some tRNAs the anticodon contains an inosine. The heterocyclic base in inosine is hypoxanthine. Inosine can form hydrogen-bonded base pairs with A, U, or C. This means that the inosine in tRNA can...
-
Use the information from BE9-19 except thatJulip Corporation is a private enterprise that applies ASPE. Prepare Julips 2014 entries to record all transactions and events related to its significant...
-
a) Provide a brief background of the Honda Motor company and industry, then identify a current ethical issue that has an effect on the industry that the Honda Motor company is operating in. 1b)...
-
If you own, did you consider leasing? If yes, why did you choose a purchase over a lease? If you lease, why did you go with a lease? List the specific advantages you feel you gained by leasing. If...
-
If 5 people worked in a process for 8 hours, which included a 0.5 hour break, and they produced 900 units. What was the worker hours per unit? from below: 2.7 minutes 24 minutes 2.5 minutes 22.5...
-
12. Access the following PIDS using the scan tool and verify current input signal status and record the status below? PIDS APP1 APP2 ECT IAT MAF RPM TP1 TP2 VSS Signal Status WSM Specifications
-
With Twitter being on the verge of bankruptcy and undergoing mass resignation, what are some creative and relative directions managers of the social media platform could take to improve the brand?
-
Tybee Industries Inc. uses a job order cost system. The following data summarize the operations related to production for January 2016, the first month of operations: a. Materials purchased on...
-
Uniform electric field in Figure a uniform electric field is directed out of the page within a circular region of radius R = 3.00 cm. The magnitude of the electric field is given by E = (4.50 x 10-3...
-
State whether you would expect each of the following properties to be identical or different for the two enantiomers of 2-pentanol. Explain. (a) Boiling point (b) Optical rotation (c) Solubility in...
-
Draw a structure for each of the following compounds in its more stable chair conformation. Explain your choice. (a) (b) CH3 CH3 CH (CH),C CHA CH, ," CH3 CH3
-
The standard free-energy difference between the two chair conformations of isopropylcyclohexane is 9.2 kJ mol-1 (2.2 kcal mol-1;. What is the ratio of concentrations of the two confirmations at 25C?
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


