Invoking Hammonds postulate and the properties of the carbocation intermediates, explain why the doubly allylic alkyl halide
Question:
Invoking Hammond’s postulate and the properties of the carbocation intermediates, explain why the doubly allylic alkyl halide A undergoes much more rapid solvolysis in aqueous acetone than compound B. Then explain why compound C, which is also a doubly allylic alkyl halide, is solvolytically inert.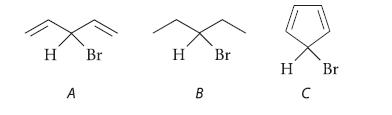
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





