Ions as well as neutral molecules undergo pericyclic reactions. Classify the pericyclic reactions of the cation involved
Question:
Ions as well as neutral molecules undergo pericyclic reactions. Classify the pericyclic reactions of the cation involved in the trans formation shown in Fig. P28.49. Tell whether the methyl groups are cis or trans and why.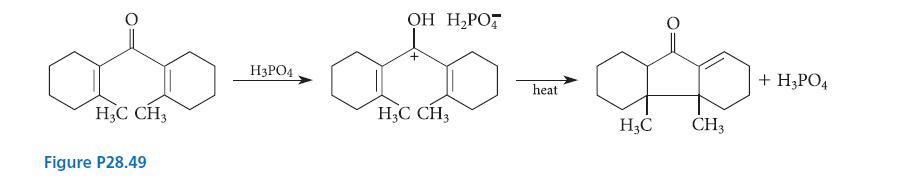
Transcribed Image Text:
овом ободат H3PO4 heat CH3 Figure P28.49 OH CH3 CH3 + H3PO4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
A thermal electrocyclic reaction of the carbocation which is formed by protonation of the ketone s...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Classify each of the following pericyclic reactions as an electrocyclic, cycloaddition, or sig- matropic reaction. Give the curved-arrow notation for each reaction, and tell how many electrons are...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
A typical steroid skeleton is shown along with the numbering scheme used for this class of compounds. Specify in each case whether the designated substituent is axial or equatorial. (a) Substituent...
-
Northwest Building Products (NBP) manufactures two lumber products from a joint milling process: residential building lumber (RBL) and commercial building lumber (CBL). A standard production run...
-
A recent annual report for FedEx included the following note: Property and Equipment Expenditures for major additions, improvements, flight equipment modifications, and certain equipment overhaul...
-
Referring to Tables 3.2 and 3.3, do you recognize any laws or regulatory agencies that would have jurisdiction over your type of product?
-
Calculate LCVs for the following information: Standard hours: 40 at Rs 3 per hour Actual hours: 50 at Rs 4 per hour
-
Twin-Cities, Inc., purchased a building for $400,000. Straight-line depreciation was used for each of the first two years using the following assumptions: 25-year estimated useful life, with a...
-
there are 2 parts to this question. thank you Homework: Chapter 17 Homework Question 1, S17-3 (simila... Part 1 of 2 Analyze the following T-accounts to determine the amount of direct and indirect...
-
In 1985, two researchers at the University of California, Riverside, carried out the reaction given in Fig. P28.51. The equilibrium mixture contained compound A (22%), a single stereoisomer of B...
-
(a) The transformation shown in Fig. P28.50, which involves a sequence of two pericyclic reactions, was used as a key step in a synthesis of the sex hormone estrone. Identify the unstable...
-
The ledger of Tombert Company has the Following work in process account Production records show that there were 700 units in the beginning inventory, 30% complete, 1,100 units started, and 1,300...
-
I have attached a case study, primarily based on your textbook chapter reading assignments. The background material for the case also references chapters 3 and 15, not assigned for this course....
-
On December 1 , 2 0 2 5 , Sandhill Distributing Company had the following account balances.DebitCash$ 7 , 1 0 0 Accounts Receivable 4 , 5 0 0 Inventory 1 1 , 9 0 0 Supplies 1 , 2 0 0 Equipment 2 2 ,...
-
Cindy Greene works at Georgia Mountain Hospital. The hospital experiences a lot of business closer to summer when the temperature is warmer. Cindy is meeting with her supervisor to go over the budget...
-
Use z scores to compare the given values. Based on sample data, newborn males have weights with a mean of 3247.4 g and a standard deviation of 575.4 g. Newborn females have weights with a mean of...
-
Gignment FULL SCAL Exercise 4- The following ndependent situations require professional judgment for determining when to recognize revenue from the transactions. Identify when revenue should be...
-
Lo-bed Company produced 4,000 units that require two standard gallons per unit at $20.00 standard price per gallon. The company actually used 8,200 gallons in production. Journalize the entry to...
-
Is that Yelp review real or fake? The article A Framework for Fake Review Detection in Online Consumer Electronics Retailers (Information Processing and Management 2019: 12341244) tested five...
-
Starting with diethyl a-bromomalonate and potassium phthalimide and using any other necessary reagents, show how you might synthesize (a) dl-leucine, (b) dl-alanine, and (c) dl-phenylalanine.
-
(a) Outline a Strecker synthesis of dl-phenylalanine. (b) dl-Methionine can also be synthesized by a Strecker synthesis. The required starting aldehyde can be prepared from acrolein (CH2 == CHCHO)...
-
The electron-withdrawing property of the 2, 4-dinitrophenyl group makes separation of the labeled amino acid very easy. Suggest how this is done.
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


