In 1985, two researchers at the University of California, Riverside, carried out the reaction given in Fig.
Question:
In 1985, two researchers at the University of California, Riverside, carried out the reaction given in Fig. P28.51.
The equilibrium mixture contained compound A (22%), a single stereoisomer of B (47%), and a single stereoisomer of C (31%). Predict the stereochemistry of compounds B and C at the carbon marked with the asterisk (*). Explain your prediction.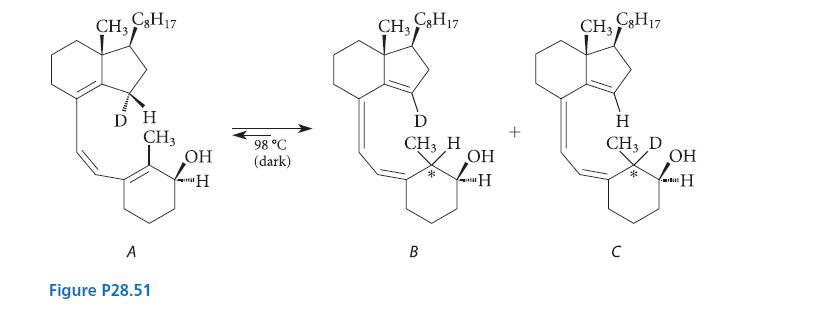
Transcribed Image Text:
CH₂ C3H17 DH A CH3 Figure P28.51 OH 'H 98 °C (dark) CH₂ C8H17 D CH3 H B * OH "H t CH₂ C8H17 H CH3 D C * OH H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
This is a 17 sigmatropic rearrangement which according to ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In 1985, two researchers at the University of California, Riverside, carried out the reaction given in Fig. P27.51. The equilibrium mixture contained compound A (22%), a single stereoisomer of B...
-
Read the case study and answer the question below with a one page response. What does a SWOT analysis reveal about the overall attractiveness of Under Armours situation? Founded in 1996 by former...
-
Researchers at the University of Utah carried out a study to see if the size of the fork used to eat dinner has an effect on how much food is consumed (Food Network Magazine, January 2012). The...
-
At fiscal year-end December 31, 2015, Shop-World had the following assets and liabilities on its balance sheet (in millions): Current liabilities ............ $9,459 Long-term debt .................
-
At the beginning of the year, Plummer's Sports Center bought three used fitness machines from Advantage, Inc. The machines immediately were overhauled, installed, and started operating. The machines...
-
Mary Rhodes, operations manager at Burnaby Furniture, has received the following estimates of demand requirements: a) Assuming stockout costs for lost sales of $100 per unit, inventory carrying costs...
-
From the particulars given, calculate the following material variances and give their relationships: (1) MCV, (2) MUV, , (3) MPV, (4) MMV and (5) material sub-usage variance Material Standard Actual...
-
For the data in exercise 14.4, use an a of 0.1 to make a forecast for July. 14.6. 14.7. 14.8. 14.9.
-
Darrin Glauser is putting away money in a vacation fund to travel in 9 years and currently has an amount of $39.420.00 Given an interest rate of 700% compounded to annually, how much will they have...
-
An interesting heterocyclic compound C was prepared and trapped by the sequence of reactions given in Fig. P28.52. Give the structure of all missing compounds, and explain what happens in each...
-
Ions as well as neutral molecules undergo pericyclic reactions. Classify the pericyclic reactions of the cation involved in the trans formation shown in Fig. P28.49. Tell whether the methyl groups...
-
Joanna, age 44, defers $23,000 in a qualified Solo 401(k) plan in 2018. a. What amount must be returned to Joanna and by what date? b. In what year will the amount be taxed? c. What percent of the...
-
Notation Using the weights (Ib) and highway fuel consumption amounts (mi/gal) of the 48 cars listed in Data Set 35 "Car Data" of Appendix B, we get this regression equation: = 58.9 - 0.00749x, where...
-
Week 11-Final Exam: Chapters 5-7 Question 15 of 30 -135 Current At in Ppm 06-20 10%.onthe 1110077 OORE Textbook and M DOLL F T 19 19 Q w A R T Y 3 . 9 4 S D 4 G H A L x N M Cu T
-
We have two samples: sample 1 n= 39 -X= 98.2 S= 15.9 sample 2 n=31 -X=119.2 S= 23.0 begin testing whether u1
-
Discuss charitable purpose trusts under Section 3(1), Charities Act 2011.
-
Amadeus Corporation is considering the issue of a new product to be added to its product mix. They hired you, a recent business graduate from MacEwan, for conducting the analysis. The production line...
-
Prepare a 2016 income statement through gross profit for Lo-bed Company, using the variance data in Practice Exercises -1A, -2A, -3A, and -4A. Assume Lo-bed sold 4,000 units at $250 per unit.
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
Bradykinin is a nonapeptide released by blood plasma globulins in response to a wasp sting. It is a very potent pain-causing agent. Its constituent amino acids are 2R, G, 2F, 3P, S. The use of 2,...
-
Complete hydrolysis of a heptapeptide showed that it has the following constituent amino acids: 2A, E, L, K, F, V Deduce the amino acid sequence of this heptapeptide from the following data. 1....
-
Part of the evidence for restricted rotation about the carbon-nitrogen bond in a peptide linkage comes from 1H NMR studies done with simple amides. For example, at room temperature the 1H NMR...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


