An interesting heterocyclic compound C was prepared and trapped by the sequence of reactions given in Fig.
Question:
An interesting heterocyclic compound C was prepared and trapped by the sequence of reactions given in Fig. P28.52.
Give the structure of all missing compounds, and explain what happens in each reaction.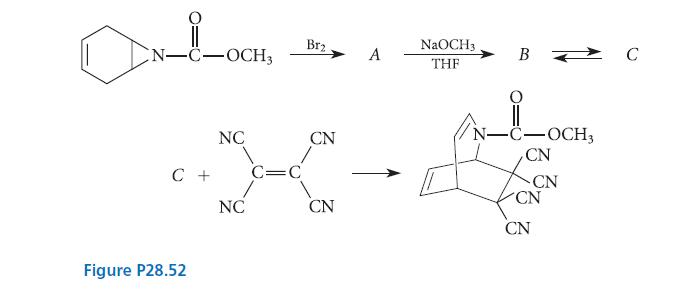
Transcribed Image Text:
N-C-OCH3 NC C + C=C Figure P28.52 NC Br₂ CN CN A NaOCH 3 THF B -C-OCH3 CN CN CN CN C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
First deduce the structure of compound C from the structu...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Celestolide, a perfuming agent with a musk odor, is prepared by the sequence of reactions given in Fig. P16.52. (a) Give the curved-arrow mechanism for the formation of compound A (reaction a). Fig....
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
The glycine cleavage system is a group of four enzymes that together catalyze the following reaction: Use the following information to determine the sequence of reactions involved in the glycine...
-
In its processing of peanuts this month, Whispering added $3,275 of DM from its warehouse directly into the roasting process, where there was an existing beginning WIP Inventory balance of $1,475....
-
Best Buy Co., Inc., headquartered in Richfield, Minnesota, is one of the leading consumer electronics retailers, operating more than 1,000 stores in the United States, Europe, Canada, China, and...
-
Using the data from Problem C.15 and the unit production costs in the following table, show which locations yield the lowest cost. In problem C.15 Drew Rosen Corp. is considering adding a fourth...
-
Calculate MPV, MUV and MCV from the following information: Quantity of materials purchased 3,000 units Value of materials purchased Rs 14,000 SQ of material required per tonne of finished product 20...
-
Suppose the scores earned on Professor McArthurs third statistics exam are normally distributed with mean 64 and standard deviation 8. Professor McArthur wants to curve the exam scores as follows:...
-
Helg Foreign exchange rates fluctuate due to changes in all but which of the following? Multiple Choice Economic conditions Political conditions. Whether the companies prepare financial statements...
-
Anticipating the isolation of the potentially aromatic hydrocarbon B, a group of chemists irradiated compound A with ultraviolet light. Compound C was obtained as a product instead of B. (a) Explain...
-
In 1985, two researchers at the University of California, Riverside, carried out the reaction given in Fig. P28.51. The equilibrium mixture contained compound A (22%), a single stereoisomer of B...
-
If the price of copper in Europe is 2.12 per ounce, what is the expected price of copper in the United States if the spot exchange rate is $1 = 0.7623?
-
Zephyr Minerals completed the following transactions involving machinery. Machine No. 1550 was purchased for cash on April 1, 2020, at an installed cost of $75,000. Its useful life was estimated to...
-
Kelly is a self-employed tax attorney whose practice primarily involves tax planning. During the year, she attended a three-day seminar regarding new changes to the tax law. She incurred the...
-
At a recently concluded Annual General Meeting (AGM) of a company, one of the shareholders remarked; historical financial statements are essential in corporate reporting, particularly for compliance...
-
4. In hypothesis, Mr. Ng wants to compare the solution in Q3 to other solutions in different conditions. If the following constraints are newly set in place, answer how much different is going to be...
-
3C2H6O2+7H2O= C2H4O3+11H2+O2+H2C2O4+CH2O2 Glycolic acid is produced electrochemically from ethylene glycol under alkaline conditions(NaOH). Hydrogen is produced at the cathode, and formic acid and...
-
Prepare a 2016 income statement through gross profit for Dvorak Company, using the variance data in Practice Exercises -1B, -2B, -3B, and -4B. Assume Dvorak sold 1,000 units at $90 per unit.
-
What is the difference between the straight-line method of depreciation and the written down value method? Which method is more appropriate for reporting earnings?
-
The guanidino group of arginine is one of the most strongly basic of all organic groups. Explain. NH NI NI
-
(a) dl-Glutamic acid has been synthesized from diethyl acetamidomalonate in the following way. Outline the reactions involved. (b) Compound G has also been used to prepare the amino acid dl-ornithine...
-
Synthetic polyglutamic acid exists as an a helix in solution at pH 2-3. When the pH of such a solution is gradually raised through the addition of a base, a dramatic change in optical rotation takes...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


